Route of Vaccine Administration Alters Antigen Trafficking but Not Innate or Adaptive Immunity
- PMID: 32209459
- PMCID: PMC7198771
- DOI: 10.1016/j.celrep.2020.02.111
Route of Vaccine Administration Alters Antigen Trafficking but Not Innate or Adaptive Immunity
Abstract
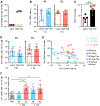
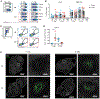
Although intramuscular (i.m.) administration is the most commonly used route for licensed vaccines, subcutaneous (s.c.) delivery is being explored for several new vaccines under development. Here, we use rhesus macaques, physiologically relevant to humans, to identify the anatomical compartments and early immune processes engaged in the response to immunization via the two routes. Administration of fluorescently labeled HIV-1 envelope glycoprotein trimers displayed on liposomes enables visualization of targeted cells and tissues. Both s.c. and i.m. routes induce efficient immune cell infiltration, activation, and antigen uptake, functions that are tightly restricted to the skin and muscle, respectively. Antigen is also transported to different lymph nodes depending on route. However, these early differences do not translate into significant differences in the magnitude or quality of antigen-specific cellular and humoral responses over time. Thus, although some distinct immunological differences are noted, the choice of route may instead be motivated by clinical practicality.
Keywords: B cell follicle; HIV envelope glycoprotein; antigen transport; dendritic cell; follicular dendritic cell; intramuscular; lymph node; monocytes; subcutaneous; vaccination.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures


References
-
- Cook IF, Barr I, Hartel G, Pond D, and Hampson AW (2006). Reactogenicity and immunogenicity of an inactivated influenza vaccine administered by intramuscular or subcutaneous injection in elderly adults. Vaccine 24, 2395–2402. - PubMed
-
- Diez-Domingo J, Weinke T, Garcia de Lomas J, Meyer CU, Bertrand I, Eymin C, Thomas S, and Sadorge C (2015). Comparison of intramuscular and subcutaneous administration of a herpes zoster live-attenuated vaccine in adults aged R50 years: a randomised non-inferiority clinical trial. Vaccine 33, 789–795. - PubMed
-
- Douagi I, Forsell MNE, Sundling C, O’Dell S, Feng Y, Dosenovic P, Li Y, Seder R, Loré K, Mascola JR, et al. (2010). Influence of novel CD4 binding-defective HIV-1 envelope glycoprotein immunogens on neutralizing antibody and T-cell responses in nonhuman primates. J. Virol 84, 1683–1695. - PMC - PubMed
-
- Fisch A, Cadilhac P, Vidor E, Prazuck T, Dublanchet A, and Lafaix C (1996). Immunogenicity and safety of a new inactivated hepatitis A vaccine: a clinical trial with comparison of administration route. Vaccine 14, 1132–1136. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

