Cardiovascular risk factors and outcomes in early rheumatoid arthritis: a population-based study
- PMID: 32209618
- PMCID: PMC7525791
- DOI: 10.1136/heartjnl-2019-316193
Cardiovascular risk factors and outcomes in early rheumatoid arthritis: a population-based study
Abstract
Objective: To assess the burden of cardiovascular disease (CVD) at and prior to diagnosis in people with early rheumatoid arthritis (RA) and subsequent CVD in these patients.
Methods: A retrospective case-control study using a large English primary care database. People with RA (n=6591) diagnosed between 2004 and 2016 (inclusive) were identified using a validated algorithm, matched 1:1 by age and gender to those without RA (n=6591) and followed for a median of 5.4 years. We assessed differences in CVD at, before and after diagnosis, and the impact of traditional and RA-related risk factors (C reactive protein, RA-related autoantibodies and medication use) on incident CVD (a composite of myocardial infarction (MI), stroke or heart failure).
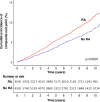
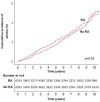
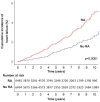
Results: RA cases and their matched controls were both of mean age 58.7 (SD 15.5) at cohort entry, and 67.5% were female. Some CVD risk factors were more common at RA diagnosis including smoking and diabetes; however, total and low-density lipoprotein cholesterol were lower in patients with RA. CVD was more common in RA at cohort entry; stroke (3.9% vs 2.7%, p<0.001), heart failure (1.6% vs 1.0%, p=0.001), and non-significantly MI (3.1% vs 2.8%, p=0.092). Excess CVD developed in the 5 years preceding diagnosis. After adjustment for traditional and RA-related risk factors, RA was associated with greater risk of post-diagnosis CVD (HR 1.33, 95% CI 1.07 to 1.65, p=0.010).
Conclusions: An excess of stroke and heart failure occurs before diagnosis of RA. There is excess risk for further cardiovascular events after diagnosis, which is not explained by differences in traditional CVD or RA-related risk factors at diagnosis.
Keywords: electronic medical records; epidemiology; statistics and study design.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: EN has received speaker honoraria and has participated in advisory boards for Pfizer, Sanofi, 110 Gilead, Celltrion, AbbVie and Lilly. SdL declares no conflicts of interest. CDM declares no conflicts of interest. KK is an employee of Pfizer. GB is an employee of Pfizer. CDB declares no conflicts of interest. JG has received honoraria and/or sponsorships for conferences from AbbVie, Celgene, Janssen, Pfizer and UCB. KR has received research funding from AbbVie and Pfizer and honoraria/consultancy fees from AbbVie, Sanofi, Lilly, Bristol-Myers Squibb, UCB, Pfizer, Janssen and Roche Chugai.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
