Negative pressure wound therapy compared with standard moist wound care on diabetic foot ulcers in real-life clinical practice: results of the German DiaFu-RCT
- PMID: 32209619
- PMCID: PMC7202734
- DOI: 10.1136/bmjopen-2018-026345
Negative pressure wound therapy compared with standard moist wound care on diabetic foot ulcers in real-life clinical practice: results of the German DiaFu-RCT
Abstract
Objectives: The aim of the DiaFu study was to evaluate effectiveness and safety of negative pressure wound therapy (NPWT) in patients with diabetic foot wounds in clinical practice.
Design: In this controlled clinical superiority trial with blinded outcome assessment patients were randomised in a 1:1 ratio stratified by study site and ulcer severity grade using a web-based-tool.
Setting: This German national study was conducted in 40 surgical and internal medicine inpatient and outpatient facilities specialised in diabetes foot care.
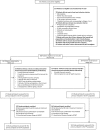
Participants: 368 patients were randomised and 345 participants were included in the modified intention-to-treat (ITT) population. Adult patients suffering from a diabetic foot ulcer at least for 4 weeks and without contraindication for NPWT were allowed to be included.
Interventions: NPWT was compared with standard moist wound care (SMWC) according to local standards and guidelines.
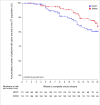
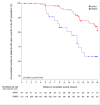
Primary and secondary outcome measures: Primary outcome was wound closure within 16 weeks. Secondary outcomes were wound-related and treatment-related adverse events (AEs), amputations, time until optimal wound bed preparation, wound size and wound tissue composition, pain and quality of life (QoL) within 16 weeks, and recurrences and wound closure within 6 months.
Results: In the ITT population, neither the wound closure rate (difference: n=4 (2.5% (95% CI-4.7% - 9.7%); p=0.53)) nor the time to wound closure (p=0.244) was significantly different between the treatment arms. 191 participants (NPWT 127; SMWC 64) had missing endpoint documentations, premature therapy ends or unauthorised treatment changes. 96 participants in the NPWT arm and 72 participants in the SMWC arm had at least one AE (p=0.007), but only 16 AEs were related to NPWT.
Conclusions: NPWT was not superior to SMWC in diabetic foot wounds in German clinical practice. Overall, wound closure rate was low. Documentation deficits and deviations from treatment guidelines negatively impacted the outcome wound closure.
Trial registration numbers: NCT01480362 and DRKS00003347.
Keywords: benefit assessment; diabetic foot; negative pressure wound therapy; wound care; wound healing; wound treatment.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: The German statutory health insurance companies commissioned the Witten/Herdecke University (UW/H) to plan, conduct, analyse and publish the study. DS is an employee of the UW/H. The study has been financed by the manufacturers KCI (Acelity) and S&N. DS received a consulting fee for the presentation of the study during an event organised by the manufacturer Hartmann. During study planning and conduct, EN was an employee of the UW/H. He was the director of the Institut für Forschung in der Operativen Medizin. The clinical investigators MS, HL, GW, PM, DH, WW-R, KS, MH, GR, TK and KZ received a case fee of 1000€ for each patient included in the DiaFu study in order to compensate for the additional organisational and especially the documentation effort during trial conduct. Furthermore, all investigators received compensation for travelling to the investigator meetings. The institutions of the investigators used integrated care contracts for NPWT during study conduct in order to provide best practice for the study participants during outpatient care. GW and WW-R are members of the scientific advisory board of the manufacturer KCI (now Acelity).
Figures


References
-
- World Health Organization Global report on diabetes, 2016. Available: http://www.who.int/diabetes/global-report/en/
-
- International Diabetes Federation IDF diabetes atlas, 2015. Available: www.diabetesatlas.org
-
- Leone S, Pascale R, Vitale M, et al. [Epidemiology of diabetic foot]. Infez Med 2012;20 Suppl 1:8–13. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
