microRNA-15b contributes to depression-like behavior in mice by affecting synaptic protein levels and function in the nucleus accumbens
- PMID: 32209659
- PMCID: PMC7242712
- DOI: 10.1074/jbc.RA119.012047
microRNA-15b contributes to depression-like behavior in mice by affecting synaptic protein levels and function in the nucleus accumbens
Abstract
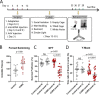
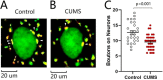
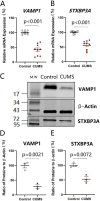
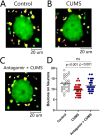
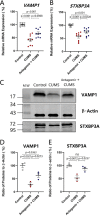
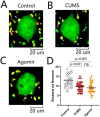
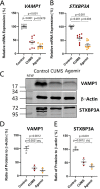
Major depression is a prevalent affective disorder characterized by recurrent low mood. It presumably results from stress-induced deteriorations of molecular networks and synaptic functions in brain reward circuits of genetically-susceptible individuals through epigenetic processes. Epigenetic regulator microRNA-15b inhibits neuronal progenitor proliferation and is up-regulated in the medial prefrontal cortex of mice that demonstrate depression-like behavior, indicating the contribution of microRNA-15 to major depression. Using a mouse model of major depression induced by chronic unpredictable mild stress (CUMS), here we examined the effects of microRNA-15b on synapses and synaptic proteins in the nucleus accumbens of these mice. The application of a microRNA-15b antagomir into the nucleus accumbens significantly reduced the incidence of CUMS-induced depression and reversed the attenuations of excitatory synapse and syntaxin-binding protein 3 (STXBP3A)/vesicle-associated protein 1 (VAMP1) expression. In contrast, the injection of a microRNA-15b analog into the nucleus accumbens induced depression-like behavior as well as attenuated excitatory synapses and STXBP3A/VAMP1 expression similar to the down-regulation of these processes induced by the CUMS. We conclude that microRNA-15b-5p may play a critical role in chronic stress-induced depression by decreasing synaptic proteins, innervations, and activities in the nucleus accumbens. We propose that the treatment of anti-microRNA-15b-5p may convert stress-induced depression into resilience.
Keywords: fusion protein; microRNA (miRNA); neuroscience; stress; synapse.
© 2020 Guo et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures






Similar articles
-
Small Differences and Big Changes: The Many Variables of MicroRNA Expression and Function in the Brain.J Neurosci. 2024 Aug 7;44(32):e0365242024. doi: 10.1523/JNEUROSCI.0365-24.2024. J Neurosci. 2024. PMID: 39111834 Free PMC article. Review.
-
microRNA and mRNA profiles in nucleus accumbens underlying depression versus resilience in response to chronic stress.Am J Med Genet B Neuropsychiatr Genet. 2018 Sep;177(6):563-579. doi: 10.1002/ajmg.b.32651. Epub 2018 Aug 14. Am J Med Genet B Neuropsychiatr Genet. 2018. PMID: 30105773 Free PMC article.
-
The molecular mechanism underlying GABAergic dysfunction in nucleus accumbens of depression-like behaviours in mice.J Cell Mol Med. 2019 Oct;23(10):7021-7028. doi: 10.1111/jcmm.14596. Epub 2019 Aug 20. J Cell Mol Med. 2019. PMID: 31430030 Free PMC article.
-
microRNA and mRNA profiles in the amygdala are associated with stress-induced depression and resilience in juvenile mice.Psychopharmacology (Berl). 2019 Jul;236(7):2119-2142. doi: 10.1007/s00213-019-05209-z. Epub 2019 Mar 21. Psychopharmacology (Berl). 2019. PMID: 30900007
-
A trace of silence: memory and microRNA at the synapse.Curr Opin Neurobiol. 2006 Oct;16(5):535-9. doi: 10.1016/j.conb.2006.08.007. Epub 2006 Sep 7. Curr Opin Neurobiol. 2006. PMID: 16962314 Review.
Cited by
-
Prokineticin-2 Participates in Chronic Constriction Injury-Triggered Neuropathic Pain and Anxiety via Regulated by NF-κB in Nucleus Accumbens Shell in Rats.Mol Neurobiol. 2024 May;61(5):2764-2783. doi: 10.1007/s12035-023-03680-6. Epub 2023 Nov 7. Mol Neurobiol. 2024. PMID: 37934398
-
Early Life Irradiation-Induced Hypoplasia and Impairment of Neurogenesis in the Dentate Gyrus and Adult Depression Are Mediated by MicroRNA- 34a-5p/T-Cell Intracytoplasmic Antigen-1 Pathway.Cells. 2021 Sep 18;10(9):2476. doi: 10.3390/cells10092476. Cells. 2021. PMID: 34572124 Free PMC article.
-
Small Differences and Big Changes: The Many Variables of MicroRNA Expression and Function in the Brain.J Neurosci. 2024 Aug 7;44(32):e0365242024. doi: 10.1523/JNEUROSCI.0365-24.2024. J Neurosci. 2024. PMID: 39111834 Free PMC article. Review.
-
A New Player in Depression: MiRNAs as Modulators of Altered Synaptic Plasticity.Int J Mol Sci. 2022 Apr 20;23(9):4555. doi: 10.3390/ijms23094555. Int J Mol Sci. 2022. PMID: 35562946 Free PMC article. Review.
-
The important roles of microRNAs in depression: new research progress and future prospects.J Mol Med (Berl). 2021 May;99(5):619-636. doi: 10.1007/s00109-021-02052-8. Epub 2021 Feb 27. J Mol Med (Berl). 2021. PMID: 33641067 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

