Efficacy and safety of recombinant human follicle-stimulating hormone in patients undergoing in vitro fertilization-embryo transfer
- PMID: 32209728
- PMCID: PMC7138541
- DOI: 10.18632/aging.102919
Efficacy and safety of recombinant human follicle-stimulating hormone in patients undergoing in vitro fertilization-embryo transfer
Erratum in
-
Correction for: Efficacy and safety of recombinant human follicle-stimulating hormone in patients undergoing in vitro fertilization-embryo transfer.Aging (Albany NY). 2023 Jun 29;15(12):5951-5953. doi: 10.18632/aging.204490. Epub 2023 Jun 29. Aging (Albany NY). 2023. PMID: 37384564 Free PMC article. No abstract available.
Abstract
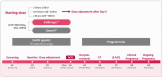
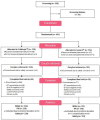
To compare the ovarian responses after administration of two recombinant follicle-stimulating hormone (r-FSH) preparations under gonadotropin-releasing hormone (GnRH) analogue downregulation, we conducted a phase 3, randomized, multicenter, assessor-blind, active-controlled, parallel group study. The primary outcome was the number of oocytes retrieved. The secondary outcomes included total dose and duration of r-FSH administered, oocyte quality, blood estradiol levels, follicular development, fertilization rates, implantation rates, and pregnancy rates (biochemical, clinical, and ongoing). A total of 451 patients with infertility were randomized to receive either Follitrope™ Prefilled Syringe or Gonal-F® Pen for ovarian stimulation. The mean number of oocytes retrieved was 14.9 in the FollitropeTM Prefilled Syringe group, and 12.8 in the Gonal-F® Pen group. The 95% confidence interval in the oocyte number difference between the groups was [-0.1, 4.2], demonstrating that FollitropeTM Prefilled Syringe was not inferior to Gonal-F® Pen. The clinical pregnancy rates (FollitropeTM Prefilled Syringe vs. Gonal-F® Pen: 55.4% vs. 51.9%) and ongoing pregnancy rates (44.1% vs. 43.0%) were similar between the groups. No clinically significant adverse events were observed in either group. In summary, our study indicates that FollitropeTM Prefilled Syringe is safe and efficacious for ovarian stimulation.
Keywords: assisted reproductive technology (ART); oocytes retrieved, IVF-ET; ovarian stimulation; recombinant human FSH (r-hFSH).
Conflict of interest statement
Figures
References
-
- Zegers-Hochschild F, Adamson GD, de Mouzon J, Ishihara O, Mansour R, Nygren K, Sullivan E, Vanderpoel S, and International Committee for Monitoring Assisted Reproductive Technology, and World Health Organization. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril. 2009; 92:1520–24. 10.1016/j.fertnstert.2009.09.009 - DOI - PubMed
-
- Donini P, Montezemolo R. Rassegna di clinica, Terapia e scienze affini. A Publ Biol Lab Inst Serono. 1949; 48:3–28.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources