VLA-4 Expression and Activation in B Cell Malignancies: Functional and Clinical Aspects
- PMID: 32210016
- PMCID: PMC7139737
- DOI: 10.3390/ijms21062206
VLA-4 Expression and Activation in B Cell Malignancies: Functional and Clinical Aspects
Abstract
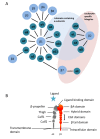
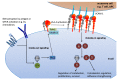
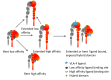
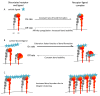
Lineage commitment and differentiation of hematopoietic cells takes place in well-defined microenvironmental surroundings. Communication with other cell types is a vital prerequisite for the normal functions of the immune system, while disturbances in this communication support the development and progression of neoplastic disease. Integrins such as the integrin very late antigen-4 (VLA-4; CD49d/CD29) control the localization of healthy as well as malignant B cells within the tissue, and thus determine the patterns of organ infiltration. Malignant B cells retain some key characteristics of their normal counterparts, with B cell receptor (BCR) signaling and integrin-mediated adhesion being essential mediators of tumor cell homing, survival and proliferation. It is thus not surprising that targeting the BCR pathway using small molecule inhibitors has proved highly effective in the treatment of B cell malignancies. Attenuation of BCR-dependent lymphoma-microenvironment interactions was, in this regard, described as a main mechanism critically contributing to the efficacy of these agents. Here, we review the contribution of VLA-4 to normal B cell differentiation on the one hand, and to the pathophysiology of B cell malignancies on the other hand. We describe its impact as a prognostic marker, its interplay with BCR signaling and its predictive role for novel BCR-targeting therapies, in chronic lymphocytic leukemia and beyond.
Keywords: B cell differentiation; B cell receptor; Bruton’s tyrosine kinase; CD49d; CLL; adhesion; chronic lymphocytic leukemia; integrin; leukemia; lymphoma; therapy; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

