TGF-β Signaling
- PMID: 32210029
- PMCID: PMC7175140
- DOI: 10.3390/biom10030487
TGF-β Signaling
Abstract
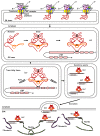
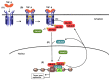
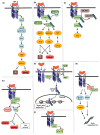
Transforming growth factor-β (TGF-β) represents an evolutionarily conserved family of secreted polypeptide factors that regulate many aspects of physiological embryogenesis and adult tissue homeostasis. The TGF-β family members are also involved in pathophysiological mechanisms that underlie many diseases. Although the family comprises many factors, which exhibit cell type-specific and developmental stage-dependent biological actions, they all signal via conserved signaling pathways. The signaling mechanisms of the TGF-β family are controlled at the extracellular level, where ligand secretion, deposition to the extracellular matrix and activation prior to signaling play important roles. At the plasma membrane level, TGF-βs associate with receptor kinases that mediate phosphorylation-dependent signaling to downstream mediators, mainly the SMAD proteins, and mediate oligomerization-dependent signaling to ubiquitin ligases and intracellular protein kinases. The interplay between SMADs and other signaling proteins mediate regulatory signals that control expression of target genes, RNA processing at multiple levels, mRNA translation and nuclear or cytoplasmic protein regulation. This article emphasizes signaling mechanisms and the importance of biochemical control in executing biological functions by the prototype member of the family, TGF-β.
Keywords: SMAD; extracellular matrix; phosphorylation; receptor serine/threonine kinase; signal transduction; transcription; transforming growth factor-β; ubiquitylation.
Conflict of interest statement
The authors declare no conflict of interest
Figures



References
-
- Derynck R., Miyazono K. The TGF-β Family. Cold Spring Harbor Laboratory Press; Woodbury, NY, USA: 2008. pp. 1–1114.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

