Subcutaneous Immunization of Leishmania HSP70-II Null Mutant Line Reduces the Severity of the Experimental Visceral Leishmaniasis in BALB/c Mice
- PMID: 32210040
- PMCID: PMC7157689
- DOI: 10.3390/vaccines8010141
Subcutaneous Immunization of Leishmania HSP70-II Null Mutant Line Reduces the Severity of the Experimental Visceral Leishmaniasis in BALB/c Mice
Abstract
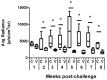
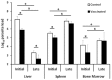
Leishmania infantum parasites cause a severe form of visceral leishmaniasis in human and viscerocutaneous leishmaniasis in dogs. Recently, we reported that immunization with an attenuated L. infantum cell line, lacking the hsp70-II gene, protects against the development of murine cutaneous leishmaniasis. In this work, we analyzed the vaccine potential of this cell line towards the long-term protection against murine visceral leishmaniasis. This model shows an organ-dependent evolution of the disease. The infection can resolve in the liver but chronically affect spleen and bone marrow. Twelve weeks after subcutaneous administration of attenuated L. infantum, Bagg Albino (BALB/c) mice were challenged with infective L. infantum parasites expressing the luciferase-encoding gene. Combining in vivo bioimaging techniques with limiting dilution experiments, we report that, in the initial phase of the disease, vaccinated animals presented lower parasite loads than unvaccinated animals. A reduction of the severity of liver damage was also detected. Protection was associated with the induction of rapid parasite-specific IFN-γ production by CD4+ and CD8+ T cells. However, the vaccine was unable to control the chronic phase of the disease, since we did not find differences in the parasite burdens nor in the immune response at that time point.
Keywords: IFN-γ; T-cells; attenuated parasites; mice; vaccine; visceral leishmaniasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Buckingham-Jeffery E., Hill E.M., Datta S., Dilger E., Courtenay O. Spatio-temporal modelling of Leishmania infantum infection among domestic dogs: A simulation study and sensitivity analysis applied to rural Brazil. Parasit. Vectors. 2019;12:215. doi: 10.1186/s13071-019-3430-y. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

