BMP-SMAD1/5 Signaling Regulates Retinal Vascular Development
- PMID: 32210087
- PMCID: PMC7175193
- DOI: 10.3390/biom10030488
BMP-SMAD1/5 Signaling Regulates Retinal Vascular Development
Abstract
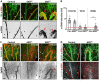
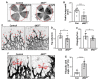
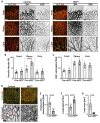
Vascular development is an orchestrated process of vessel formation from pre-existing vessels via sprouting and intussusceptive angiogenesis as well as vascular remodeling to generate the mature vasculature. Bone morphogenetic protein (BMP) signaling via intracellular SMAD1 and SMAD5 effectors regulates sprouting angiogenesis in the early mouse embryo, but its role in other processes of vascular development and in other vascular beds remains incompletely understood. Here, we investigate the function of SMAD1/5 during early postnatal retinal vascular development using inducible, endothelium-specific deletion of Smad1 and Smad5. We observe the formation of arterial-venous malformations in areas with high blood flow, and fewer and less functional tip cells at the angiogenic front. The vascular plexus region is remarkably hyperdense and this is associated with reduced vessel regression and aberrant vascular loop formation. Taken together, our results highlight important functions of SMAD1/5 during vessel formation and remodeling in the early postnatal retina.
Keywords: BMP signaling; SMAD1/5; arteriovenous malformations; intussusceptive angiogenesis; retina development; sprouting angiogenesis; vessel regression.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Lee H.W., Chong D.C., Ola R., Dunworth W.P., Meadows S., Ka J., Kaartinen V.M., Qyang Y., Cleaver O., Bautch V.L., et al. Alk2/ACVR1 and Alk3/BMPR1A Provide Essential Function for Bone Morphogenetic Protein-Induced Retinal Angiogenesis. Arter. Thromb. Vasc. Biol. 2017;37:657–663. doi: 10.1161/ATVBAHA.116.308422. - DOI - PMC - PubMed
-
- Ola R., Kunzel S.H., Zhang F., Genet G., Chakraborty R., Pibouin-Fragner L., Martin K., Sessa W., Dubrac A., Eichmann A. SMAD4 Prevents Flow Induced Arteriovenous Malformations by Inhibiting Casein Kinase 2. Circulation. 2018;138:2379–2394. doi: 10.1161/CIRCULATIONAHA.118.033842. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

