Ultrasensitive detection of nucleic acids using deformed graphene channel field effect biosensors
- PMID: 32210235
- PMCID: PMC7093535
- DOI: 10.1038/s41467-020-15330-9
Ultrasensitive detection of nucleic acids using deformed graphene channel field effect biosensors
Abstract
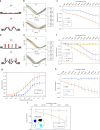
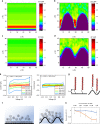
Field-effect transistor (FET)-based biosensors allow label-free detection of biomolecules by measuring their intrinsic charges. The detection limit of these sensors is determined by the Debye screening of the charges from counter ions in solutions. Here, we use FETs with a deformed monolayer graphene channel for the detection of nucleic acids. These devices with even millimeter scale channels show an ultra-high sensitivity detection in buffer and human serum sample down to 600 zM and 20 aM, respectively, which are ∼18 and ∼600 nucleic acid molecules. Computational simulations reveal that the nanoscale deformations can form 'electrical hot spots' in the sensing channel which reduce the charge screening at the concave regions. Moreover, the deformed graphene could exhibit a band-gap, allowing an exponential change in the source-drain current from small numbers of charges. Collectively, these phenomena allow for ultrasensitive electronic biomolecular detection in millimeter scale structures.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

