A Staphylococcus pro-apoptotic peptide induces acute exacerbation of pulmonary fibrosis
- PMID: 32210242
- PMCID: PMC7093394
- DOI: 10.1038/s41467-020-15344-3
A Staphylococcus pro-apoptotic peptide induces acute exacerbation of pulmonary fibrosis
Abstract
Idiopathic pulmonary fibrosis (IPF) is a chronic and fatal disease of unknown etiology; however, apoptosis of lung alveolar epithelial cells plays a role in disease progression. This intractable disease is associated with increased abundance of Staphylococcus and Streptococcus in the lungs, yet their roles in disease pathogenesis remain elusive. Here, we report that Staphylococcus nepalensis releases corisin, a peptide conserved in diverse staphylococci, to induce apoptosis of lung epithelial cells. The disease in mice exhibits acute exacerbation after intrapulmonary instillation of corisin or after lung infection with corisin-harboring S. nepalensis compared to untreated mice or mice infected with bacteria lacking corisin. Correspondingly, the lung corisin levels are significantly increased in human IPF patients with acute exacerbation compared to patients without disease exacerbation. Our results suggest that bacteria shedding corisin are involved in acute exacerbation of IPF, yielding insights to the molecular basis for the elevation of staphylococci in pulmonary fibrosis.
Conflict of interest statement
C.N.D.G: and E.C.G. have a patent on the TGFβ1 TG mice used in the present study. There is an invention disclosure by C.N.D.G:, E.C.G., and I.C. on the apoptotic peptides identified in this study. None of the other authors declared any conflict of interest regarding the present work.
Figures
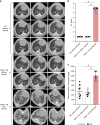


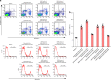
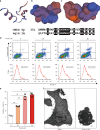
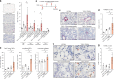
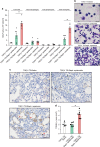
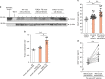
References
-
- King TE, Jr., Noble PW, Bradford WZ. Treatments for idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014;371:783–784. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

