The Human Lung Glycome Reveals Novel Glycan Ligands for Influenza A Virus
- PMID: 32210305
- PMCID: PMC7093477
- DOI: 10.1038/s41598-020-62074-z
The Human Lung Glycome Reveals Novel Glycan Ligands for Influenza A Virus
Abstract
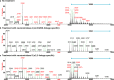
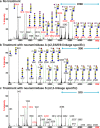
Glycans within human lungs are recognized by many pathogens such as influenza A virus (IAV), yet little is known about their structures. Here we present the first analysis of the N- and O- and glycosphingolipid-glycans from total human lungs, along with histological analyses of IAV binding. The N-glycome of human lung contains extremely large complex-type N-glycans with linear poly-N-acetyllactosamine (PL) [-3Galβ1-4GlcNAcβ1-]n extensions, which are predominantly terminated in α2,3-linked sialic acid. By contrast, smaller N-glycans lack PL and are enriched in α2,6-linked sialic acids. In addition, we observed large glycosphingolipid (GSL)-glycans, which also consists of linear PL, terminating in mainly α2,3-linked sialic acid. Histological staining revealed that IAV binds to sialylated and non-sialylated glycans and binding is not concordant with respect to binding by sialic acid-specific lectins. These results extend our understanding of the types of glycans that may serve as binding sites for human lung pathogens.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Varki, A. Essentials of glycobiology. Third edition/Edited by Ajit Varki et al. Cold Spring Harbor Press (2017).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

