Differences in Net Information Flow and Dynamic Connectivity Metrics Between Physically Active and Inactive Subjects Measured by Functional Near-Infrared Spectroscopy (fNIRS) During a Fatiguing Handgrip Task
- PMID: 32210748
- PMCID: PMC7076120
- DOI: 10.3389/fnins.2020.00167
Differences in Net Information Flow and Dynamic Connectivity Metrics Between Physically Active and Inactive Subjects Measured by Functional Near-Infrared Spectroscopy (fNIRS) During a Fatiguing Handgrip Task
Abstract
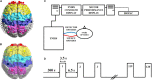
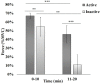
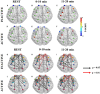
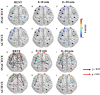
Twenty-three young adults (4 Females, 25.13 ± 3.72 years) performed an intermittent maximal handgrip force task using their dominant hand for 20 min (3.5 s squeeze/6.5 s release, 120 blocks) with concurrent cortical activity imaging by functional Near-Infrared Spectroscopy (fNRIS; OMM-3000, Shimadzu Corp., 111 channels). Subjects were grouped as physically active (n = 10) or inactive (n = 12) based on a questionnaire (active-exercise at least four times a week, inactive- exercise less than two times a week). We explored how motor task fatigue affected the vasomotion-induced oscillations in ΔHbO as measured by fNIRS at each hemodynamic frequency band: endothelial component (0.003-0.02 Hz) associated to microvascular activity, neurogenic component (0.02-0.04 Hz) related to intrinsic neuronal activity, and myogenic component (0.04-0.15 Hz) linked to activity of smooth muscles of arterioles. To help understand how these three neurovascular regulatory mechanisms relate to handgrip task performance we quantified several dynamic fNIRS metrics, including directional phase transfer entropy (dPTE), a computationally efficient and data-driven method used as a marker of information flow between cortical regions, and directional connectivity (DC), a means to compute directionality of information flow between two cortical regions. The relationship between static functional connectivity (SFC) and functional connectivity variability (FCV) was also explored to understand their mutual dependence for each frequency band in the context of handgrip performance as fatigued increased. Our findings ultimately showed differences between subject groups across all fNIRS metrics and hemodynamic frequency bands. These findings imply that physical activity modulates neurovascular control mechanisms at the endogenic, neurogenic, and myogenic frequency bands resulting in delayed fatigue onset and enhanced performance. The dynamic cortical network metrics quantified in this work for young, healthy subjects provides baseline measurements to guide future work on older individuals and persons with impaired cardiovascular health.
Keywords: directional connectivity; directional phase transfer entropy; fatigue; functional connectivity variability; sensory-motor cortex.
Copyright © 2020 Urquhart, Wang, Liu, Fadel and Alexandrakis.
Figures

References
-
- Andersen A. V., Simonsen S. A., Schytz H. W., Iversen H. K. (2018). Assessing low-frequency oscillations in cerebrovascular diseases and related conditions with near-infrared spectroscopy: a plausible method for evaluating cerebral autoregulation? Neurophotonics 5:030901. 10.1117/1.NPh.5.3.030901 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

