CD8 T-cell Recruitment Into the Central Nervous System of Cuprizone-Fed Mice: Relevance to Modeling the Etiology of Multiple Sclerosis
- PMID: 32210765
- PMCID: PMC7076139
- DOI: 10.3389/fncel.2020.00043
CD8 T-cell Recruitment Into the Central Nervous System of Cuprizone-Fed Mice: Relevance to Modeling the Etiology of Multiple Sclerosis
Abstract
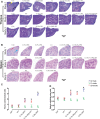
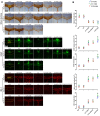
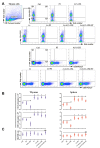
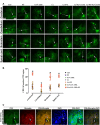
Cuprizone (CPZ)-feeding in mice induces atrophy of peripheral immune organs (thymus and spleen) and suppresses T-cell levels, thereby limiting its use as a model for studying the effects of the immune system in demyelinating diseases such as Multiple Sclerosis (MS). To investigate whether castration (Cx) can protect the peripheral immune organs from CPZ-induced atrophy and enable T-cell recruitment into the central nervous system (CNS) following a breach of the blood-brain barrier (BBB), three related studies were carried out. In Study 1, Cx prevented the dose-dependent reductions (0.1% < 0.2% CPZ) in thymic and splenic weight, size of the thymic medulla and splenic white pulp, and CD4 and CD8 (CD4/8) levels remained comparable to gonadally intact (Gi) control males. Importantly, 0.1% and 0.2% CPZ were equipotent at inducing central demyelination and glial activation. In Study 2, combining Cx with 0.1% CPZ-feeding and BBB disruption with pertussis toxin (PT) enhanced CD8+ T-cell recruitment into the CNS. The increased CD8+ T-cell level observed in the parenchyma of the cerebrum, cerebellum, brainstem and spinal cord were confirmed by flow cytometry and western blot analyses of CNS tissue. In Study 3, PT+0.1% CPZ-feeding to Gi female mice resulted in similar effects on the peripheral immune organs, CNS demyelination, and gliosis comparable to Gi males, indicating that testosterone levels alone were not responsible for the immune response seen in Study 2. The combination of Cx+0.1% CPZ-feeding+PT indicates that CPZ-induced demyelination can trigger an "inside-out" immune response when the peripheral immune system is spared and may provide a better model to study the initiating events in demyelinating conditions such as MS.
Keywords: atrophy; castration; demyelination; gliosis; gonadally intact; inside-out; peripheral immune organs.
Copyright © 2020 Almuslehi, Sen, Shortland, Mahns and Coorssen.
Figures




References
-
- Alt C., Laschinger M., Engelhardt B. (2002). Functional expression of the lymphoid chemokines CCL19 (ELC) and CCL 21 (SLC) at the blood-brain barrier suggests their involvement in G-protein-dependent lymphocyte recruitment into the central nervous system during experimental autoimmune encephalomyelitis. Eur. J. Immunol. 32, 2133–2144. 10.1002/1521-4141(200208)32:8<2133::aid-immu2133>3.0.co;2-w - DOI - PubMed
-
- Azar A., Rank M. A., Zarka M. A., Chang Y. H., Lal D. (2017). Gender-specific differences in serum immunoglobulin E levels and prevalence of fungus in sinonasal tissue noted in patients with chronic rhinosinusitis who underwent endoscopic sinus surgery. Am. J. Rhinol. Allergy 31, 370–375. 10.2500/ajra.2017.31.4471 - DOI - PubMed
-
- Babbe H., Roers A., Waisman A., Lassmann H., Goebels N., Hohlfeld R., et al. . (2000). Clonal expansions of CD8(+) T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J. Exp. Med. 192, 393–404. 10.1084/jem.192.3.393 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

