Contra-Directional Expression of Plasma Superoxide Dismutase with Lipoprotein Cholesterol and High-Sensitivity C-reactive Protein as Important Markers of Parkinson's Disease Severity
- PMID: 32210787
- PMCID: PMC7068795
- DOI: 10.3389/fnagi.2020.00053
Contra-Directional Expression of Plasma Superoxide Dismutase with Lipoprotein Cholesterol and High-Sensitivity C-reactive Protein as Important Markers of Parkinson's Disease Severity
Abstract
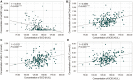
Aim: Oxidative stress and inflammation play critical roles in the neuropathogenesis of PD. We aimed to evaluate oxidative stress and inflammation status by measuring serum superoxide dismutase (SOD) with lipoprotein cholesterol and high-sensitivity C-reactive protein (hsCRP) respectively in PD patients, and explore their correlation with the disease severity. Methods: We performed a cross-sectional study that included 204 PD patients and 204 age-matched healthy controls (HCs). Plasma levels of SOD, hsCRP, total cholesterol, high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) were measured. A series of neuropsychological assessments were performed to rate the severity of PD. Results: The plasma levels of SOD (135.7 ± 20.14 vs. 147.2 ± 24.34, P < 0.0001), total cholesterol, HDL-C and LDL-C in PD were significantly lower than those in HCs; the hsCRP level was remarkably increased in PD compared to HC (2.766 ± 3.242 vs. 1.637 ± 1.597, P < 0.0001). The plasma SOD was negatively correlated with the hsCRP, while positively correlated with total cholesterol, HDL-C, and LDL-C in PD patients. The plasma SOD were negatively correlated with H&Y, total UPDRS, UPDRS (I), UPDRS (II), and UPDRS (III) scores, but positively correlated with MoCA and MMSE scores. Besides, hsCRP was negatively correlated with MoCA; while total cholesterol, HDL-C and LDL-C were positively correlated with the MoCA, respectively. Conclusion: Our findings suggest that lower SOD along with cholesterol, HDL-C and LDL-C, and higher hsCRP levels might be important markers to assess the PD severity. A better understanding of SOD and hsCRP may yield insights into the pathogenesis of PD.
Keywords: Parkinson’s disease; high-sensitivity C-reactive protein; inflammation; lipoprotein cholesterol; oxidative stress; superoxide dismutase.
Copyright © 2020 Yang, Chang, Que, Weng, Deng, Wang, Huang, Xie, Wei, Yang, Li, Ma, Zhou, Tang, Mok, Zhu and Wang.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials

