Functional Selectivity and Antinociceptive Effects of a Novel KOPr Agonist
- PMID: 32210803
- PMCID: PMC7066533
- DOI: 10.3389/fphar.2020.00188
Functional Selectivity and Antinociceptive Effects of a Novel KOPr Agonist
Abstract
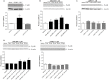
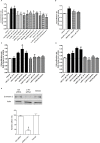
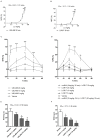
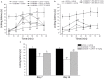
Kappa opioid receptor (KOPr) agonists represent alternative analgesics for their low abuse potential, although relevant adverse effects have limited their clinical use. Functionally selective KOPr agonists may activate, in a pathway-specific manner, G protein-mediated signaling, that produces antinociception, over β-arrestin 2-dependent induction of p38MAPK, which preferentially contributes to adverse effects. Thus, functionally selective KOPr agonists biased toward G protein-coupled intracellular signaling over β-arrestin-2-mediated pathways may be considered candidate therapeutics possibly devoid of many of the typical adverse effects elicited by classic KOPr agonists. Nonetheless, the potential utility of functionally selective agonists at opioid receptors is still highly debated; therefore, further studies are necessary to fully understand whether it will be possible to develop more effective and safer analgesics by exploiting functional selectivity at KOPr. In the present study we investigated in vitro functional selectivity and in vivo antinociceptive effects of LOR17, a novel KOPr selective peptidic agonist that we synthesized. LOR17-mediated effects on adenylyl cyclase inhibition, ERK1/2, p38MAPK phosphorylation, and astrocyte cell proliferation were studied in HEK-293 cells expressing hKOPr, U87-MG glioblastoma cells, and primary human astrocytes; biased agonism was investigated via cAMP ELISA and β-arrestin 2 recruitment assays. Antinociception and antihypersensitivity were assessed in mice via warm-water tail-withdrawal test, intraperitoneal acid-induced writhing, and a model of oxaliplatin-induced neuropathic cold hypersensitivity. Effects of LOR17 on locomotor activity, exploratory activity, and forced-swim behavior were also assayed. We found that LOR17 is a selective, G protein biased KOPr agonist that inhibits adenylyl cyclase and activates early-phase ERK1/2 phosphorylation. Conversely to classic KOPr agonists as U50,488, LOR17 neither induces p38MAPK phosphorylation nor increases KOPr-dependent, p38MAPK-mediated cell proliferation in astrocytes. Moreover, LOR17 counteracts, in a concentration-dependent manner, U50,488-induced p38MAPK phosphorylation and astrocyte cell proliferation. Both U50,488 and LOR17 display potent antinociception in models of acute nociception, whereas LOR17 counteracts oxaliplatin-induced thermal hypersensitivity better than U50,488, and it is effective after single or repeated s.c. administration. LOR17 administered at a dose that fully alleviated oxaliplatin-induced thermal hypersensitivity did not alter motor coordination, locomotor and exploratory activities nor induced pro-depressant-like behavior. LOR17, therefore, may emerge as a novel KOPr agonist displaying functional selectivity toward G protein signaling and eliciting antinociceptive/antihypersensitivity effects in different animal models, including oxaliplatin-induced neuropathy.
Keywords: antinociception; chemotherapy-induced neuropathic pain; functional selectivity; intracellular signaling; kappa opioid receptor.
Copyright © 2020 Bedini, Di Cesare Mannelli, Micheli, Baiula, Vaca, De Marco, Gentilucci, Ghelardini and Spampinato.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

