Nrf2-SHP Cascade-Mediated STAT3 Inactivation Contributes to AMPK-Driven Protection Against Endotoxic Inflammation
- PMID: 32210977
- PMCID: PMC7076194
- DOI: 10.3389/fimmu.2020.00414
Nrf2-SHP Cascade-Mediated STAT3 Inactivation Contributes to AMPK-Driven Protection Against Endotoxic Inflammation
Abstract
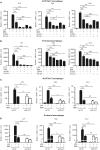
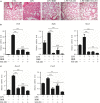
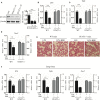
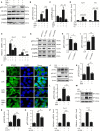
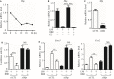
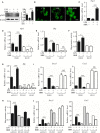
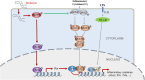
Signal transducer and activator of transcription 3 (STAT3) is implicated in inflammation processing, but the mechanism of its regulation mostly remains limited to Janus kinase (JAK)-mediated phosphorylation. Although AMP-activated protein kinase (AMPK)-mediated STAT3 inactivation has got documented, the molecular signaling cascade connecting STAT3 inactivation and the anti-inflammatory role of AMPK is far from established. In the present study, we addressed the interplay between AMPK and STAT3, and revealed the important role of STAT3 inactivation in the anti-inflammatory function of AMPK in lipopolysaccharide-stressed macrophages and mice. Firstly, we found that pharmacological inhibition of STAT3 can improve the anti-inflammatory effect of AMPK in wild-type mice, and the expression of STAT3 in macrophage of mice is a prerequisite for the anti-inflammatory effect of AMPK. As to the molecular signaling cascade linking AMPK to STAT3, we disclosed that AMPK suppressed STAT3 not only by attenuating JAK signaling but also by activating nuclear factor erythroid-2-related factor-2 (Nrf2), a redox-regulating transcription factor, which consequently increased the expression of small heterodimer protein (SHP), thus repressing the transcriptional activity of STAT3. In summary, this study provided a unique set of evidence showing the relationship between AMPK and STAT3 signaling and explored a new mechanism of AMPK-driven STAT3 inactivation that involves Nrf2-SHP signaling cascade. These findings expand our understanding of the interplay between pro- and anti-inflammatory signaling pathways and are beneficial for the therapeutic development of sepsis treatments.
Keywords: AMPK; LPS; Nrf2; SHP; STAT3; inflammation.
Copyright © 2020 Gong, Tai, Huang, Xiao, Mo, Wang, Han, Zhou, Chen, Tang, Zhao, Xu, Gong, Zhang, Yang, Wang and Xiao.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

