A New Remote Sensing-Based System for the Monitoring and Analysis of Growth and Gas Exchange Rates of Photosynthetic Microorganisms Under Simulated Non-Terrestrial Conditions
- PMID: 32210991
- PMCID: PMC7066451
- DOI: 10.3389/fpls.2020.00182
A New Remote Sensing-Based System for the Monitoring and Analysis of Growth and Gas Exchange Rates of Photosynthetic Microorganisms Under Simulated Non-Terrestrial Conditions
Abstract
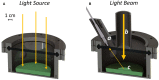
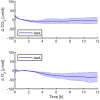
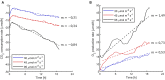
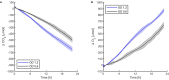
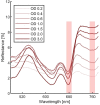
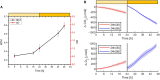
Oxygenic photosynthetic microorganisms are a focal point of research in the context of human space exploration. As part of the bioregenerative life-support systems, they could have a key role in the production of breathable O2, edible biomasses and in the regeneration of CO2 rich-atmospheres and wastewaters produced by astronauts. The test of the organism's response to simulated physico-chemical parameters of planetary bodies could also provide important information about their habitability potential. It is believed that the success of future planetary and space missions will require innovative technologies, developed on the base of preliminary experiments in custom-made laboratory facilities. In this context, simulation chambers will play a pivotal role by allowing the growth of the microorganisms under controlled conditions and the evaluation in real-time of their biomass productivity and impact on atmosphere composition. We here present a system capable of addressing these requirements with high replicability and low costs. The setup is composed by three main parts: 1) a Star Light Simulator, able to generate different light intensities and spectra, including those of non-solar stars; 2) an Atmosphere Simulator Chamber where cultures of photosynthetic microorganisms can be exposed to different gas compositions; 3) a reflectivity detection system to measure from remote the Normalized Difference Vegetation Indexes (NDVI). Such a setup allows us to monitor photosynthetic microorganism's growth and gas exchange performances under selected conditions of light quality and intensity, temperature, pressure, and atmospheres simulating non-terrestrial environments. All parameters are detected by remote sensing techniques, thus without interfering with the experiments and altering the environmental conditions set. We validated the setup by growing cyanobacteria liquid cultures under different light intensities of solar illumination, collecting data on their growth rate, photosynthetic activity, and gas exchange capacity. We utilized the reflectivity detection system to measure the reflection spectra of the growing cultures, obtaining their relative NDVI that was shown to correlate with optical density, chlorophyll content, and dry weight, demonstrating the potential application of this index as a proxy of growth.
Keywords: Normalized Difference Vegetation Indexes; cyanobacteria; photosynthesis; reflectance spectra; remote sensing; simulation chamber.
Copyright © 2020 Battistuzzi, Cocola, Salasnich, Erculiani, Alei, Morosinotto, Claudi, Poletto and La Rocca.
Figures



References
-
- Billi D., Baqué M., Smith H. D., McKay C. P. (2013). Cyanobacteria from extreme deserts to space. Adv. Microbiol. 03, 80–86. 10.4236/aim.2013.36a010 - DOI
-
- Claudi R., Erculiani M. S., Galletta G., Billi D., Pace E., Schierano D., et al. (2015). Simulating super earth atmospheres in the laboratory. Int. J. Astrobiol. 15, 35–44. 10.1017/S1473550415000117 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

