1D Copper(II)-Aroylhydrazone Coordination Polymers: Magnetic Properties and Microwave Assisted Oxidation of a Secondary Alcohol
- PMID: 32211380
- PMCID: PMC7069101
- DOI: 10.3389/fchem.2020.00157
1D Copper(II)-Aroylhydrazone Coordination Polymers: Magnetic Properties and Microwave Assisted Oxidation of a Secondary Alcohol
Abstract
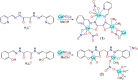
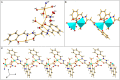
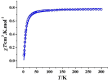
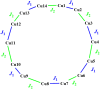
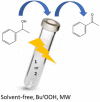
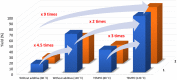
The 1D Cu(II) coordination polymers [Cu3(L1)(NO3)4(H2O)2]n (1) and [Cu2(H2L2)(NO3)(H2O)2]n(NO3)n (2) have been synthesized using the aroylhyrazone Schiff bases N' 1,N' 2-bis(pyridin-2-ylmethylene)oxalohydrazide (H2L1) and N' 1,N' 3-bis(2-hydroxybenzylidene)malonohydrazide (H4L2), respectively. They have been characterized by elemental analysis, infrared (IR) spectroscopy, UV-Vis spectroscopy, electrospray ionization mass spectrometry (ESI-MS), single crystal X-ray diffraction and variable temperature magnetic susceptibility measurements (for 2). The ligand (L1)2- coordinates in the iminol form in 1, whereas the amide coordination is observed for (H2L2)2- in 2. Either the ligand bridge or the nitrate bridge in 2 mediates weak antiferromagnetic coupling. The catalytic performance of 1 and 2 has been investigated toward the solvent-free microwave-assisted oxidation of a secondary alcohol (1-phenylethanol used as model substrate). At 120°C and in the presence of the nitroxyl radical 2,2,6,6-tetramethylpiperydil-1-oxyl (TEMPO), the complete conversion of 1-phenylethanol into acetophenone occurs with TOFs up to 1,200 h-1.
Keywords: Cu(II) complexes; X-ray structure; coordination polymer; magnetism; microwave assisted oxidation of alcohols.
Copyright © 2020 Sutradhar, Alegria, Barman, Guedes da Silva, Liu and Pombeiro.
Figures


References
-
- Ahmad J. U., Figiel P. J., Räisänen M. T., Leskelä M., Repo T. (2009). Aerobic oxidation of benzylic alcohols with bis(3,5-di-tert-butylsalicylaldimine)copper(II) complexes. Appl. Catal. A 371, 17–21. 10.1016/j.apcata.2009.09.011 - DOI
-
- Andruh M., Branzea G. D., Gheorghe R., Madalan A. M. (2009). Crystal engineering of hybrid inorganic–organic systems based upon complexes with dissymmetric compartmental ligands. Cryst. Eng. Comm. 11, 2571–2584. 10.1039/b909476h - DOI
-
- Bruker (2012). APEX2. Madison, Wisconsin: Bruker AXS Inc.,
LinkOut - more resources
Full Text Sources
Miscellaneous

