Clinical Outcomes of Soft Tissue Preservation Surgery With Hydroxyapatite-Coated Abutments Compared to Traditional Percutaneous Bone Conduction Hearing Implant Surgery-A Pragmatic Multi-Center Randomized Controlled Trial
- PMID: 32211417
- PMCID: PMC7066494
- DOI: 10.3389/fsurg.2020.00005
Clinical Outcomes of Soft Tissue Preservation Surgery With Hydroxyapatite-Coated Abutments Compared to Traditional Percutaneous Bone Conduction Hearing Implant Surgery-A Pragmatic Multi-Center Randomized Controlled Trial
Abstract
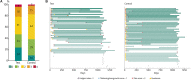
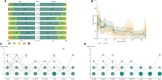
Background: Soft tissue preservation using a hydroxyapatite-coated abutment in bone conduction hearing implant surgery may lead to improved clinical outcomes over the short (1 year) and long term (3 years). Methods: In this open multi-center, randomized (1:1), controlled clinical trial, subjects with conductive, mixed hearing loss or single-sided sensorineural deafness were randomly assigned to receive the conventional intervention, a titanium abutment with soft tissue reduction surgery (control), or a new intervention, a hydroxyapatite-coated abutment with soft tissue preservation surgery (test). The primary efficacy outcome was the combined endpoint of numbness, pain, peri-abutment dermatitis, and soft tissue thickening/overgrowth after 1 and 3 years. Results: The Intention-to-treat (ITT) population consisted of 52 control subjects and 51 test subjects. The difference between the groups after 1 year of follow-up as measured by the primary efficacy outcome was not statistically significant (p = 0.12) in the ITT population (n = 103), but did reach statistical significance (p = 0.03) in the per-protocol (PP) population (n = 96). It showed an advantage for the test group, with over twice as many subjects (29%) without these medical events during the first year compared to the control group (13%). After 3 years, the difference between the two groups had declined and did not reach statistical significance (24 vs. 10%, ITT p = 0.45). Secondary outcome measures which showed a statistical significant difference during the first year, such as surgical time (15 vs. 25 minutes, p < 0.0001), numbness (90 vs. 69% of subjects experienced no numbness at 1 year, p < 0.01), neuropathic pain at 3 months (p = 0.0087) and the overall opinion of the esthetic outcome (observer POSAS scale at 3 months, p < 0.01) were favorable for the test group. More soft tissue thickening/overgrowth was observed at 3 weeks for the test group (p = 0.016). Similar results were achieved for the long term follow up. Conclusions: Soft tissue preservation with a hydroxyapatite-coated abutment leads to a reduction in the combined occurrence of complications over the first year which is not statistically significant in the ITT population but is in the PP population. This effect decreased for the long-term study follow up of 3 years and did also not reach statistical significance.
Keywords: BAHA; RCT - randomized controlled trial; hydroxyapatite; soft tissue preservation; surgery.
Copyright © 2020 van Hoof, Wigren, Ivarsson Blechert, Joore, Mateijsen, Bom, Stalfors, Eeg-Olofsson, Deguine, van der Rijt, Flynn, Algarra, Stokroos and the Angelfish Collaborative.
Figures





References
-
- Tjellström A, Lindström J, Hallén O, Albrektsson T, Brånemark P. Osseointegrated titanium implants in the temporal bone. A clinical study on bone-anchored hearing aids. Am J Otol. (1981) 2:304–10. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

