The Toll-Like Receptor 3 Agonist Polyriboinosinic Polyribocytidylic Acid Increases the Numbers of NK Cells with Distinct Phenotype in the Liver of B6 Mice
- PMID: 32211442
- PMCID: PMC7077049
- DOI: 10.1155/2020/2489407
The Toll-Like Receptor 3 Agonist Polyriboinosinic Polyribocytidylic Acid Increases the Numbers of NK Cells with Distinct Phenotype in the Liver of B6 Mice
Abstract
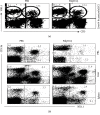
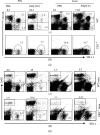
One of the activating factors of the cells of the innate immune system is the agonists of toll-like receptors (TLRs). Our earlier publications detailed how poly(I:C), a TLR3 agonist, elevates the NK cell population and the associated antigen-specific CD8+ T cell responses. This study involved a single treatment of the B6 mice with poly(I:C) intraperitoneally. To perform a detailed phenotypic analysis, mononuclear cells were prepared from each of the liver, peripheral blood, and spleen. These cells were then examined for their NK cell population by flow cytometric analysis following cell staining with indicated antibodies. The findings of the study showed that the NK cell population of the liver with an NK1.1highCD11bhighCD11chigh B220+Ly6G- phenotype was elevated following the treatment with poly(I:C). In the absence of CD11b molecule (CR3-/- mice), poly(I:C) can still increase the remained numbers of NK cells with NK1.1+CD11b- and NK1.1+Ly6G- phenotypes in the liver while their numbers in the blood decrease. After the treatment with anti-AGM1 Ab, which induced depletion of NK1.1+CD11b+ cells and partial depletion of CD3+NK1.1+ and NK1.1+CD11b- cell populations, poly(I:C) normalized the partial decreases in the numbers of NK cells concomitant with increased numbers of NK1.1-CD11b+ cell population in both liver and blood. Regarding mice with a TLR3-/- phenotype, their injection with poly(I:C) resulted in the partial elevation in the NK cell population as compared to wild-type B6 mice. To summarise, the TLR3 agonist poly(I:C) results in the elevation of a subset of liver NK cells expressing the two myeloid markers CD11c and CD11b. The effect of poly(I:C) on NK cells is partially dependent on TLR3 and independent of the presence of CD11b.
Copyright © 2020 Mohamed L. Salem et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

