Connective Tissue Remodeling in Myopia and its Potential Role in Increasing Risk of Glaucoma
- PMID: 32211567
- PMCID: PMC7093055
- DOI: 10.1016/j.cobme.2020.01.001
Connective Tissue Remodeling in Myopia and its Potential Role in Increasing Risk of Glaucoma
Abstract
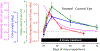
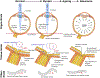
Myopia and glaucoma are both increasing in prevalence and are linked by an unknown mechanism as many epidemiologic studies have identified moderate to high myopia as an independent risk factor for glaucoma. Myopia and glaucoma are both chronic conditions that lead to connective tissue remodeling within the sclera and optic nerve head. The mechanobiology underlying connective tissue remodeling differs substantially between both diseases, with different homeostatic control mechanisms. In this article, we discuss similarities and differences between connective tissue remodeling in myopia and glaucoma; selected multi-scale mechanisms that are thought to underlie connective tissue remodeling in both conditions; how asymmetric remodeling of the optic nerve head may predispose a myopic eye for pathological remodeling and glaucoma; and how neural tissue deformations may accumulate throughout both pathologies and increase the risk for mechanical insult of retinal ganglion cell axons.
Keywords: glaucoma; mechanobiology; myopia; ocular biomechanics; optic nerve head; remodeling.
Conflict of interest statement
Declaration of interests The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


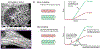
References
-
- Rudnicka AR, Kapetanakis VV, Wathern AK, Logan NS, Gilmartin B, Whincup PH, Cook DG, Owen CG, Global variations and time trends in the prevalence of childhood myopia, a systematic review and quantitative meta-analysis: implications for aetiology and early prevention., The British journal of ophthalmology 100 (2016) 882–890. doi: 10.1136/bjophthalmol-2015-307724. - DOI - PMC - PubMed
-
- Kapetanakis VV, Chan MPY, Foster PJ, Cook DG, Owen CG, Rudnicka AR, Global variations and time trends in the prevalence of primary open angle glaucoma (poag): a systematic review and meta-analysis., The British journal of ophthalmology 100 (2016) 86–93. doi: 10.1136/bjophthalmol-2015-307223. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
