The blood-brain barrier in health and disease: Important unanswered questions
- PMID: 32211826
- PMCID: PMC7144528
- DOI: 10.1084/jem.20190062
The blood-brain barrier in health and disease: Important unanswered questions
Abstract
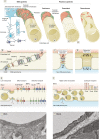
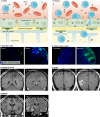
The blood vessels vascularizing the central nervous system exhibit a series of distinct properties that tightly control the movement of ions, molecules, and cells between the blood and the parenchyma. This "blood-brain barrier" is initiated during angiogenesis via signals from the surrounding neural environment, and its integrity remains vital for homeostasis and neural protection throughout life. Blood-brain barrier dysfunction contributes to pathology in a range of neurological conditions including multiple sclerosis, stroke, and epilepsy, and has also been implicated in neurodegenerative diseases such as Alzheimer's disease. This review will discuss current knowledge and key unanswered questions regarding the blood-brain barrier in health and disease.
© 2020 Profaci et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures
References
-
- Abadier M., Haghayegh Jahromi N., Cardoso Alves L., Boscacci R., Vestweber D., Barnum S., Deutsch U., Engelhardt B., and Lyck R.. 2015. Cell surface levels of endothelial ICAM-1 influence the transcellular or paracellular T-cell diapedesis across the blood-brain barrier. Eur. J. Immunol. 45:1043–1058. 10.1002/eji.201445125 - DOI - PubMed
-
- Aird W.C. 2007. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ. Res. 100:174–190. 10.1161/01.RES.0000255690.03436.ae - DOI - PubMed
-
- Alafuzoff I., Adolfsson R., Bucht G., and Winblad B.. 1983. Albumin and immunoglobulin in plasma and cerebrospinal fluid, and blood-cerebrospinal fluid barrier function in patients with dementia of Alzheimer type and multi-infarct dementia. J. Neurol. Sci. 60:465–472. 10.1016/0022-510X(83)90157-0 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

