Selective Enzymatic Oxidation of Silanes to Silanols
- PMID: 32212229
- PMCID: PMC7511438
- DOI: 10.1002/anie.202002861
Selective Enzymatic Oxidation of Silanes to Silanols
Abstract
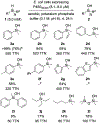
Compared to the biological world's rich chemistry for functionalizing carbon, enzymatic transformations of the heavier homologue silicon are rare. We report that a wild-type cytochrome P450 monooxygenase (P450BM3 from Bacillus megaterium, CYP102A1) has promiscuous activity for oxidation of hydrosilanes to give silanols. Directed evolution was applied to enhance this non-native activity and create a highly efficient catalyst for selective silane oxidation under mild conditions with oxygen as the terminal oxidant. The evolved enzyme leaves C-H bonds present in the silane substrates untouched, and this biotransformation does not lead to disiloxane formation, a common problem in silanol syntheses. Computational studies reveal that catalysis proceeds through hydrogen atom abstraction followed by radical rebound, as observed in the native C-H hydroxylation mechanism of the P450 enzyme. This enzymatic silane oxidation extends nature's impressive catalytic repertoire.
Keywords: P450 enzymes; biocatalysis; directed evolution; monooxygenation; silanols.
© 2020 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures



References
-
- Jeon M, Han J, Park J, ACS Catal. 2012, 2, 1539–1549;
- b) Chandrasekhar V, Boomishankar R, Nagendran S, Chem. Rev 2004, 104, 5847–5910. - PubMed
-
- Colas A, Silicones: Preparation, Properties and Performance; Dow Corning, Life Sciences, 2005.
-
-
Especially silanediols in catalysis:
Diemoz KM, Hein JE, Wilson SO, Fettinger JC, Franz AK, J. Org. Chem 2017, 82, 6738–6747
and references cited therein.
Schafer AG, Wieting JM, Fisher TJ, Mattson AE, Angew. Chem 2013, 125, 11531–11534; Angew. Chem. Int. Ed. 2013, 52, 11321–11324.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

