A Randomized Double-Blind Placebo-Controlled First-In-Human Phase 1 Trial of TransCon PTH in Healthy Adults
- PMID: 32212275
- PMCID: PMC9328939
- DOI: 10.1002/jbmr.4016
A Randomized Double-Blind Placebo-Controlled First-In-Human Phase 1 Trial of TransCon PTH in Healthy Adults
Abstract
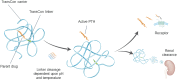
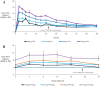
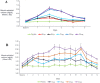
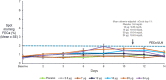
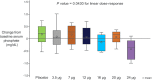
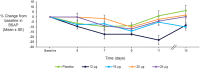
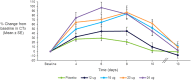
TransCon PTH is a sustained-release, essentially inactive prodrug transiently bound to an inert carrier, designed to release PTH(1-34), and in development for hypoparathyroidism (HP). This phase 1, randomized, placebo-controlled, single and multiple ascending dose (SAD and MAD, respectively) trial evaluated safety, tolerability, pharmacodynamics (PD), and pharmacokinetics (PK) of TransCon PTH in healthy adults. SAD and MAD cohorts consisted of 10 subjects (eight active, two placebo) who received up to seven single or six multiple ascending doses of TransCon PTH, respectively. TransCon PTH doses ranged from 3.5 to 124 μg PTH(1-34) for the SAD cohorts and 3.5 to 24 μg PTH(1-34)/day for the MAD cohorts. The primary PK endpoint was Free PTH. The PD endpoints included albumin adjusted serum calcium (sCa), fractional excretion of calcium (FECa), intact endogenous PTH(1-84), bone turnover markers, renal tubular maximum reabsorption of phosphate/glomerular filtration rate (TMP/GFR), serum phosphate (sP) and magnesium, and 1,25 dihydroxyvitamin D. TransCon PTH was generally well tolerated; there were no drug-related serious adverse events (SAEs), and all AEs were transient in nature. Free PTH demonstrated an effective half-life of approximately 60 hours and a dose-dependent, sustained exposure with an infusion-like profile within the calculated physiologic range for active PTH at steady-state. Albumin-adjusted sCa demonstrated a dose-dependent, sustained response with complete control of FECa despite modest hypercalcemia at higher doses. Renal tubular maximum reabsorption of phosphate/glomerular filtration rate (TMP/GFR) showed a dose-dependent decrease, resulting in a dose-dependent decrease in sP. TransCon PTH administered daily for 10 days showed no increase in the osteoblastic bone formation markers, serum bone-specific alkaline phosphatase (BSAP) or P1NP, or the osteoclastic bone resorption marker, urine NTx, but modestly and transiently increased the osteoclast marker, serum CTx. These phase 1 data support TransCon PTH as a daily replacement therapy for HP providing physiological levels of PTH 24 hours per day and advancement into phase 2 clinical development. © 2020 The Authors. Journal of Bone and Mineral Research published by American Society for Bone and Mineral Research.
Keywords: CLINICAL TRIALS; DISORDERS OF CALCIUM/PHOSPHATE METABOLISM; HYPOPARATHYROIDISM; LONG ACTING PTH; PTH; THERAPEUTICS.
© 2020 The Authors. Journal of Bone and Mineral Research published by American Society for Bone and Mineral Research.
Figures


References
-
- Mohan S, Kutilek S, Zhang C, et al. Comparison of bone formation responses to parathyroid hormone(1‐34), (1‐31), and (2‐34) in mice. Bone. 2000;27(4):471–8. - PubMed
-
- Brandi ML, Bilezikian JP, Shoback D, et al. Management of hypoparathyroidism: summary statement and guidelines. J Clin Endocrinol Metab. 2016;101(6):2273–83. - PubMed
-
- Almquist M, Ivarsson K, Nordenström E, Bergenfelz A. Mortality in patients with permanent hypoparathyroidism after total thyroidectomy. Br J Surg. 2018;105(10):1313–8. - PubMed
-
- Underbjerg L, Sikjaer T, Mosekilde L, Rejnmark L. Cardiovascular and renal complications to postsurgical hypoparathyroidism: a Danish nationwide controlled historic follow‐up study. J Bone Miner Res. 2013;28(11):2277–85. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

