mTOR signaling at the crossroads of environmental signals and T-cell fate decisions
- PMID: 32212344
- PMCID: PMC8101438
- DOI: 10.1111/imr.12845
mTOR signaling at the crossroads of environmental signals and T-cell fate decisions
Abstract
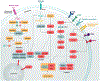
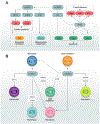
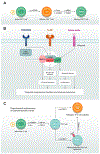
The evolutionarily conserved serine/threonine kinase mTOR (mechanistic target of rapamycin) forms the distinct protein complexes mTORC1 and mTORC2 and integrates signals from the environment to coordinate downstream signaling events and various cellular processes. T cells rely on mTOR activity for their development and to establish their homeostasis and functional fitness. Here, we review recent progress in our understanding of the upstream signaling and downstream targets of mTOR. We also provide an updated overview of the roles of mTOR in T-cell development, homeostasis, activation, and effector-cell fate decisions, as well as its important impacts on the suppressive activity of regulatory T cells. Moreover, we summarize the emerging roles of mTOR in T-cell exhaustion and transdifferentiation. A better understanding of the contribution of mTOR to T-cell fate decisions will ultimately aid in the therapeutic targeting of mTOR in human disease.
Keywords: T cell; Treg cell; iNKT cell; mTOR; metabolism.
© 2020 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI131703/AI/NIAID NIH HHS/United States
- AI1055887/Foundation for the National Institutes of Health/International
- R01 AI150514/AI/NIAID NIH HHS/United States
- R37 AI105887/AI/NIAID NIH HHS/United States
- AI150514/Foundation for the National Institutes of Health/International
- AI150241/Foundation for the National Institutes of Health/International
- AI131703/Foundation for the National Institutes of Health/International
- R01 CA221290/CA/NCI NIH HHS/United States
- R01 AI150241/AI/NIAID NIH HHS/United States
- R01 AI140761/AI/NIAID NIH HHS/United States
- CA221290/Foundation for the National Institutes of Health/International
- R01 AI105887/AI/NIAID NIH HHS/United States
- AI140761/Foundation for the National Institutes of Health/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

