PERK (Protein Kinase RNA-Like ER Kinase) Branch of the Unfolded Protein Response Confers Neuroprotection in Ischemic Stroke by Suppressing Protein Synthesis
- PMID: 32212900
- PMCID: PMC7188566
- DOI: 10.1161/STROKEAHA.120.029071
PERK (Protein Kinase RNA-Like ER Kinase) Branch of the Unfolded Protein Response Confers Neuroprotection in Ischemic Stroke by Suppressing Protein Synthesis
Abstract
Background and Purpose- Ischemic stroke impairs endoplasmic reticulum (ER) function, causes ER stress, and activates the unfolded protein response. The unfolded protein response consists of 3 branches controlled by ER stress sensor proteins, which include PERK (protein kinase RNA-like ER kinase). Activated PERK phosphorylates eIF2α (eukaryotic initiation factor 2 alpha), resulting in inhibition of global protein synthesis. Here, we aimed to clarify the role of the PERK unfolded protein response branch in stroke. Methods- Neuron-specific and tamoxifen-inducible PERK conditional knockout (cKO) mice were generated by cross-breeding Camk2a-CreERT2 with Perkf/f mice. Transient middle cerebral artery occlusion was used to induce stroke. Short- and long-term stroke outcomes were evaluated. Protein synthesis in the brain was assessed using a surface-sensing-of-translation approach. Results- After tamoxifen-induced deletion of Perk in forebrain neurons was confirmed in PERK-cKO mice, PERK-cKO and control mice were subjected to transient middle cerebral artery occlusion and 3 days or 3 weeks recovery. PERK-cKO mice had larger infarcts and worse neurological outcomes compared with control mice, suggesting that PERK-induced eIF2α phosphorylation and subsequent suppression of translation protects neurons from ischemic stress. Indeed, better stroke outcomes were observed in PERK-cKO mice that received postischemic treatment with salubrinal, which can restore the ischemia-induced increase in phosphorylated eIF2α in these mice. Finally, our data showed that post-treatment with salubrinal improved functional recovery after stroke. Conclusions- Here, we presented the first evidence that postischemic suppression of translation induced by PERK activation promotes recovery of neurological function after stroke. This confirms and further extends our previous observations that recovery of ER function impaired by ischemic stress critically contributes to stroke outcome. Therefore, future research should include strategies to improve stroke outcome by targeting unfolded protein response branches to restore protein homeostasis in neurons.
Keywords: animals; mice; neurons; neuroprotection; proteostasis.
Figures
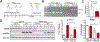

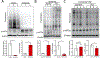


References
-
- Kleihues P, Hossmann KA. Protein synthesis in the cat brain after prolonged cerebral ischemia. Brain Res. 1971;35:409–418 - PubMed
-
- Thilmann R, Xie Y, Kleihues P, Kiessling M. Persistent inhibition of protein synthesis precedes delayed neuronal death in postischemic gerbil hippocampus. Acta Neuropathol. 1986;71:88–93 - PubMed
-
- Paschen W, Proud CG, Mies G. Shut-down of translation, a global neuronal stress response: Mechanisms and pathological relevance. Curr Pharm Des. 2007;13:1887–1902 - PubMed
-
- Cooper HK, Zalewska T, Kawakami S, Hossmann KA, Kleihues P. The effect of ischaemia and recirculation on protein synthesis in the rat brain. J Neurochem. 1977;28:929–934 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

