A Critical Role for Estrogen-Related Receptor Signaling in Cardiac Maturation
- PMID: 32212902
- PMCID: PMC7274895
- DOI: 10.1161/CIRCRESAHA.119.316100
A Critical Role for Estrogen-Related Receptor Signaling in Cardiac Maturation
Abstract
Rationale: The heart undergoes dramatic developmental changes during the prenatal to postnatal transition, including maturation of cardiac myocyte energy metabolic and contractile machinery. Delineation of the mechanisms involved in cardiac postnatal development could provide new insight into the fetal shifts that occur in the diseased heart and unveil strategies for driving maturation of stem cell-derived cardiac myocytes.
Objective: To delineate transcriptional drivers of cardiac maturation.
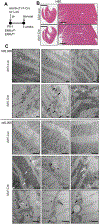
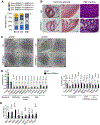
Methods and results: We hypothesized that ERR (estrogen-related receptor) α and γ, known transcriptional regulators of postnatal mitochondrial biogenesis and function, serve a role in the broader cardiac maturation program. We devised a strategy to knockdown the expression of ERRα and γ in heart after birth (pn-csERRα/γ [postnatal cardiac-specific ERRα/γ]) in mice. With high levels of knockdown, pn-csERRα/γ knockdown mice exhibited cardiomyopathy with an arrest in mitochondrial maturation. RNA sequence analysis of pn-csERRα/γ knockdown hearts at 5 weeks of age combined with chromatin immunoprecipitation with deep sequencing and functional characterization conducted in human induced pluripotent stem cell-derived cardiac myocytes (hiPSC-CM) demonstrated that ERRγ activates transcription of genes involved in virtually all aspects of postnatal developmental maturation, including mitochondrial energy transduction, contractile function, and ion transport. In addition, ERRγ was found to suppress genes involved in fibroblast activation in hearts of pn-csERRα/γ knockdown mice. Disruption of Esrra and Esrrg in mice during fetal development resulted in perinatal lethality associated with structural and genomic evidence of an arrest in cardiac maturation, including persistent expression of early developmental and noncardiac lineage gene markers including cardiac fibroblast signatures. Lastly, targeted deletion of ESRRA and ESRRG in hiPSC-CM derepressed expression of early (transcription factor 21 or TCF21) and mature (periostin, collagen type III) fibroblast gene signatures.
Conclusions: ERRα and γ are critical regulators of cardiac myocyte maturation, serving as transcriptional activators of adult cardiac metabolic and structural genes, an.d suppressors of noncardiac lineages including fibroblast determination.
Keywords: cardiomyocytes; cell differentiation; fibroblasts; genetic transcription; mitochondria; postnatal cardiac development.
Conflict of interest statement
DISCLOSURES
The authors declare no competing interests.
Figures






Comment in
-
ERRing on the Side of a Mature Heart.Circ Res. 2020 Jun 5;126(12):1703-1705. doi: 10.1161/CIRCRESAHA.120.317052. Epub 2020 Jun 4. Circ Res. 2020. PMID: 32496915 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

