Clinical outcomes with ustekinumab as rescue treatment in therapy-refractory or therapy-intolerant ulcerative colitis
- PMID: 32213052
- PMCID: PMC7006008
- DOI: 10.1177/2050640619895361
Clinical outcomes with ustekinumab as rescue treatment in therapy-refractory or therapy-intolerant ulcerative colitis
Abstract
Background: Recently, ustekinumab a monoclonal antibody targeting interleukin-12 and -23 and successfully used in Crohn's disease also has been shown to be effective in induction and maintaining remission in patients with moderate to severe ulcerative colitis in a large phase 3 trial. However, no observational data on the use of ustekinumab in ulcerative colitis in daily clinical practice is available.
Aim: The purpose of this study was to assess the clinical outcomes achieved with ustekinumab as rescue treatment in therapy-refractory or -intolerant ulcerative colitis in a real-life setting.
Methods: A retrospective data analysis was performed in 19 ulcerative colitis patients who were intolerant or refractory to all of the following drugs: steroids, purine-analogues, tumour necrosis factor antibodies and vedolizumab. To all patients ustekinumab was provided as a rescue treatment (intravenous induction with 6 mg/kg, followed by week subcutaneous injection once every eight weeks of 90 mg). The primary outcome was achievement of clinical remission at one year, defined as score of ≤ 3 points in the Lichtiger score (colitis activity index). Patients were evaluated regularly and a colonoscopy was performed before the start and at the end of the observation. Ethical approval was provided by Ethikkommission Ärztekammer Hamburg (PV 5539).
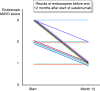
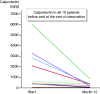
Results: In five patients, therapy was stopped due to refractory disease or side effects. In all remaining 14 patients the median colitis activity index dropped from 8.5 points (range 1-12) at start to 2.0 points at one year (range 0-5.5) and Mayo endoscopy scores fell from a median of two points (range 1-3, mean of 2.3) at start to a median of one point (range 1-3, mean of 1.4) at one year. Including the five drop-outs, clinical remission was achieved in 53% of the 19 patients at one year.
Conclusions: In accordance with the UNIFI (A Study to Evaluate the Safety and Efficacy of Ustekinumab Induction and Maintenance Therapy in Participants With Moderately to Severely Active Ulcerative Colitis) trial our real-life data support ustekinumab as an effective and safe treatment option in therapy refractory moderate to severe ulcerative colitis with a history of biological therapies.
Keywords: Colonoscopy; colon; gastroenterology; inflammatory bowel disease; surgery; ulcerative colitis; ustekinumab.
Figures


Comment in
-
Lack of evidence for the use of ustekinumab for acute severe ulcerative colitis.United European Gastroenterol J. 2021 Feb;9(1):127. doi: 10.1177/2050640620972959. Epub 2021 Feb 19. United European Gastroenterol J. 2021. PMID: 33176618 Free PMC article. No abstract available.
-
Lack of evidence for the use of ustekinumab for acute severe ulcerative colitis.United European Gastroenterol J. 2021 Mar;9(2):279. doi: 10.1002/ueg2.12052. United European Gastroenterol J. 2021. PMID: 33871925 Free PMC article. No abstract available.
References
-
- Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med 2011; 365: 1713e25–1713e25. - PubMed
-
- Parragi L, Fournier N, Zeitz J, et al. Colectomy rates in ulcerative colitis are low and decreasing: 10-Year follow-up data from the Swiss IBD cohort study. J Crohns Colitis 2018; 12: 811–818. - PubMed
-
- Murthy SK, Begum J, Benchimol EI, et al. Introduction of anti-TNF therapy has not yielded expected declines in hospitalisation and intestinal resection rates in inflammatory bowel diseases: A population-based interrupted time series study. Gut. Epub ahead of print 12 June 2019. DOI: 10.1136/gutjnl-2019-318440. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

