The complicated clinical course in a case of atypical lipodystrophy after development of neutralizing antibody to metreleptin: treatment with setmelanotide
- PMID: 32213649
- PMCID: PMC7159256
- DOI: 10.1530/EDM-19-0139
The complicated clinical course in a case of atypical lipodystrophy after development of neutralizing antibody to metreleptin: treatment with setmelanotide
Abstract
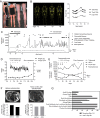
Summary: A patient with atypical partial lipodystrophy who had a transient initial response to metreleptin experienced acute worsening of her metabolic state when neutralizing antibodies against metreleptin appeared. Because her metabolic status continued to deteriorate, a therapeutic trial with melanocortin-4 receptor agonist setmelanotide, that is believed to function downstream from leptin receptor in the leptin signaling system, was undertaken in an effort to improve her metabolic status for the first time in a patient with lipodystrophy. To achieve this, a compassionate use (investigational new drug application; IND) was initiated (NCT03262610). Glucose control, body fat by dual-energy X-ray absorptiometry and MRI, and liver fat by proton density fat fraction were monitored. Daily hunger scores were assessed by patient filled questionnaires. Although there was a slight decrease in hunger scales and visceral fat, stimulating melanocortin-4 receptor by setmelanotide did not result in any other metabolic benefit such as improvement of hypertriglyceridemia or diabetes control as desired. Targeting melanocortin-4 receptor to regulate energy metabolism in this setting was not sufficient to obtain a significant metabolic benefit. However, complex features of our case make it difficult to generalize these observations to all cases of lipodystrophy. It is still possible that melanocortin-4 receptor agonistic action may offer some therapeutic benefits in leptin-deficient patients.
Learning points: A patient with atypical lipodystrophy with an initial benefit with metreleptin therapy developed neutralizing antibodies to metreleptin (Nab-leptin), which led to substantial worsening in metabolic control. The neutralizing activity in her serum persisted for longer than 3 years. Whether the worsening in her metabolic state was truly caused by the development of Nab-leptin cannot be fully ascertained, but there was a temporal relationship. The experience noted in our patient at least raises the possibility for concern for substantial metabolic worsening upon emergence and persistence of Nab-leptin. Further studies of cases where Nab-leptin is detected and better assay systems to detect and characterize Nab-leptin are needed. The use of setmelanotide, a selective MC4R agonist targeting specific neurons downstream from the leptin receptor activation, was not effective in restoring metabolic control in this complex patient with presumed diminished leptin action due to Nab-leptin. Although stimulating the MC4R pathway was not sufficient to obtain a significant metabolic benefit in lowering triglycerides and helping with her insulin resistance as was noted with metreleptin earlier, there was a mild reduction in reported food intake and appetite. Complex features of our case make it difficult to generalize our observation to all leptin-deficient patients. It is possible that some leptin-deficient patients (especially those who need primarily control of food intake) may still theoretically benefit from MC4R agonistic action, and further studies in carefully selected patients may help to tease out the differential pathways of metabolic regulation by the complex network of leptin signaling system.
Keywords: 2020; Adipose tissue; Adolescent/young adult; Alanine aminotransferase; Amenorrhoea; Anion gap; Antinuclear antibody; Apolipoprotein A-1*; Apolipoprotein B*; Appetite*; BMI; Beta-hydroxybutyrate; C-peptide (blood); Cholesterol:HDL ratio*; Cirrhosis; Complement 4*; DEXA scan; DNA sequencing; Diabetes mellitus type 1; Diabetic ketoacidosis; Diet; Fat loss*; Fatigue; Female; Fenofibrate; Fluid repletion; Food intake*; GADA; Gamma-glutamyl transpeptidase*; Glucose (blood); Haemoglobin A1c; Hand contractures*; Histopathology; Hyperglycaemia; Hyperlipidaemia*; Hypertriglyceridaemia*; Hypoglycaemia; Hypogonadism; Hypoleptinaemia*; Insulin; Insulin resistance; Ketones (plasma); Ketones (urine); Leptin; Leptin*; Lipodystrophy; Liver biopsy; Liver fat*; Liver function; MRI; March; Metformin; Metreleptin*; Mixed meal test*; Nonalcoholic steatohepatitis*; Novel treatment; Obesity; Paediatrics; Pancreatitis; Pioglitazone; Plasmapheresis; Polydipsia; Prednisone; Radioimmunoassay; Scoliosis; Setmelanotide*; Tanner scale; Thiazolidinediones; Triglycerides; United States; Visceral fat*; Vision - blurred; Weight; White.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

