Semaglutide lowers body weight in rodents via distributed neural pathways
- PMID: 32213703
- PMCID: PMC7213778
- DOI: 10.1172/jci.insight.133429
Semaglutide lowers body weight in rodents via distributed neural pathways
Abstract
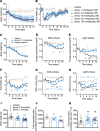
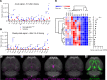
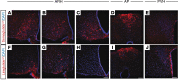
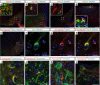
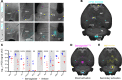
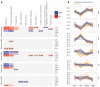
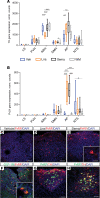
Semaglutide, a glucagon-like peptide 1 (GLP-1) analog, induces weight loss, lowers glucose levels, and reduces cardiovascular risk in patients with diabetes. Mechanistic preclinical studies suggest weight loss is mediated through GLP-1 receptors (GLP-1Rs) in the brain. The findings presented here show that semaglutide modulated food preference, reduced food intake, and caused weight loss without decreasing energy expenditure. Semaglutide directly accessed the brainstem, septal nucleus, and hypothalamus but did not cross the blood-brain barrier; it interacted with the brain through the circumventricular organs and several select sites adjacent to the ventricles. Semaglutide induced central c-Fos activation in 10 brain areas, including hindbrain areas directly targeted by semaglutide, and secondary areas without direct GLP-1R interaction, such as the lateral parabrachial nucleus. Automated analysis of semaglutide access, c-Fos activity, GLP-1R distribution, and brain connectivity revealed that activation may involve meal termination controlled by neurons in the lateral parabrachial nucleus. Transcriptomic analysis of microdissected brain areas from semaglutide-treated rats showed upregulation of prolactin-releasing hormone and tyrosine hydroxylase in the area postrema. We suggest semaglutide lowers body weight by direct interaction with diverse GLP-1R populations and by directly and indirectly affecting the activity of neural pathways involved in food intake, reward, and energy expenditure.
Keywords: Metabolism; Mouse models; Neuroimaging; Neuroscience; Obesity.
Conflict of interest statement
Figures

References
-
- van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WH. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int J Obes (Lond) 2014;38(6):784–793. doi: 10.1038/ijo.2013.162. - DOI - PMC - PubMed
-
- O’Neil PM, et al. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet. 2018;392(10148):637–649. doi: 10.1016/S0140-6736(18)31773-2. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

