Ribonuclease 1 attenuates septic cardiomyopathy and cardiac apoptosis in a murine model of polymicrobial sepsis
- PMID: 32213712
- PMCID: PMC7205433
- DOI: 10.1172/jci.insight.131571
Ribonuclease 1 attenuates septic cardiomyopathy and cardiac apoptosis in a murine model of polymicrobial sepsis
Abstract
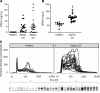
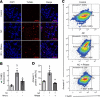
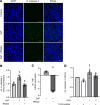
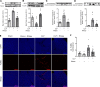
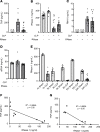
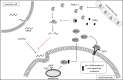
Septic cardiomyopathy is a life-threatening organ dysfunction caused by sepsis. Ribonuclease 1 (RNase 1) belongs to a group of host-defense peptides that specifically cleave extracellular RNA (eRNA). The activity of RNase 1 is inhibited by ribonuclease-inhibitor 1 (RNH1). However, the role of RNase 1 in septic cardiomyopathy and associated cardiac apoptosis is completely unknown. Here, we show that sepsis resulted in a significant increase in RNH1 and eRNA serum levels compared with those of healthy subjects. Treatment with RNase 1 resulted in a significant decrease of apoptosis, induced by the intrinsic pathway, and TNF expression in murine cardiomyocytes exposed to either necrotic cardiomyocytes or serum of septic patients for 16 hours. Additionally, treatment of septic mice with RNase 1 resulted in a reduction in cardiac apoptosis, TNF expression, and septic cardiomyopathy. These data demonstrate that eRNA plays a crucial role in the pathophysiology of the organ (cardiac) dysfunction in sepsis and that RNase and RNH1 may be new therapeutic targets and/or strategies to reduce the cardiac injury and dysfunction caused by sepsis.
Keywords: Anesthesiology; Immunology; Inflammation; Innate immunity; Pharmacology.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

