Intercellular Trafficking of Gold Nanostars in Uveal Melanoma Cells for Plasmonic Photothermal Therapy
- PMID: 32213846
- PMCID: PMC7153714
- DOI: 10.3390/nano10030590
Intercellular Trafficking of Gold Nanostars in Uveal Melanoma Cells for Plasmonic Photothermal Therapy
Abstract
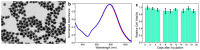
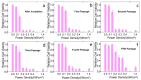
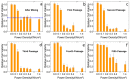
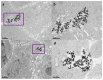
Efficient plasmonic photothermal therapies (PPTTs) using non-harmful pulse laser irradiation at the near-infrared (NIR) are a highly sought goal in nanomedicine. These therapies rely on the use of plasmonic nanostructures to kill cancer cells while minimizing the applied laser power density. Cancer cells have an unsettled capacity to uptake, retain, release, and re-uptake gold nanoparticles, thus offering enormous versatility for research. In this work, we have studied such cell capabilities for nanoparticle trafficking and its impact on the effect of photothermal treatments. As our model system, we chose uveal (eye) melanoma cells, since laser-assisted eye surgery is routinely used to treat glaucoma and cataracts, or vision correction in refractive surgery. As nanostructure, we selected gold nanostars (Au NSs) due to their high photothermal efficiency at the near-infrared (NIR) region of the electromagnetic spectrum. We first investigated the photothermal effect on the basis of the dilution of Au NSs induced by cell division. Using this approach, we obtained high PPTT efficiency after several cell division cycles at an initial low Au NS concentration (pM regime). Subsequently, we evaluated the photothermal effect on account of cell division upon mixing Au NS-loaded and non-loaded cells. Upon such mixing, we observed trafficking of Au NSs between loaded and non-loaded cells, thus achieving effective PPTT after several division cycles under low irradiation conditions (below the maximum permissible exposure threshold of skin). Our study reveals the ability of uveal melanoma cells to release and re-uptake Au NSs that maintain their plasmonic photothermal properties throughout several cell division cycles and re-uptake. This approach may be readily extrapolated to real tissue and even to treat in situ the eye tumor itself. We believe that our method can potentially be used as co-therapy to disperse plasmonic gold nanostructures across affected tissues, thus increasing the effectiveness of classic PPTT.
Keywords: femtosecond pulse laser; gold nanostars; nanoparticle endocytosis; nanoparticle exocytosis; plasmonic photothermal therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Ali M.R.K., Wu Y., El-Sayed M.A. Gold-nanoparticle-assisted plasmonic photothermal therapy advances toward clinical application. J. Phys. Chem. C. 2019;123:15375–15393. doi: 10.1021/acs.jpcc.9b01961. - DOI
-
- Guerrero-Martínez A., Barbosa S., Pastoriza-Santos I., Liz-Marzán M.L. Nanostars shine bright for you: Colloidal synthesis, properties and applications of branched metallic nanoparticles. Curr. Opin. Colloid Interface Sci. 2011;16:118–127. doi: 10.1016/j.cocis.2010.12.007. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

