The Role of the Subthalamic Nucleus in Inhibitory Control of Oculomotor Behavior in Parkinson's Disease
- PMID: 32214128
- PMCID: PMC7096507
- DOI: 10.1038/s41598-020-61572-4
The Role of the Subthalamic Nucleus in Inhibitory Control of Oculomotor Behavior in Parkinson's Disease
Abstract
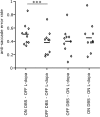
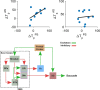
Inhibiting inappropriate actions in a context is an important part of the human cognitive repertoire, and deficiencies in this ability are common in neurological and psychiatric disorders. An anti-saccade is a simple oculomotor task that tests this ability by requiring inhibition of saccades to peripheral targets (pro-saccade) and producing voluntary eye movements toward the mirror position (anti-saccades). Previous studies provide evidence for a possible contribution from the basal ganglia in anti-saccade behavior, but the precise role of different components is still unclear. Parkinson's disease patients with implanted deep brain stimulators (DBS) in subthalamic nucleus (STN) provide a unique opportunity to investigate the role of the STN in anti-saccade behavior. Previous attempts to show the effect of STN DBS on anti-saccades have produced conflicting observations. For example, the effect of STN DBS on anti-saccade error rate is not yet clear. Part of this inconsistency may be related to differences in dopaminergic states in different studies. Here, we tested Parkinson's disease patients on anti- and pro-saccade tasks ON and OFF STN DBS, in ON and OFF dopaminergic medication states. First, STN DBS increases anti-saccade error rate while patients are OFF dopamine replacement therapy. Second, dopamine replacement therapy and STN DBS interact: L-dopa reduces the effect of STN DBS on anti-saccade error rate. Third, STN DBS induces different effects on pro- and anti-saccades in different patients. These observations provide evidence for an important role for the STN in the circuitry underlying context-dependent modulation of visuomotor action selection.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Haynes WI, Haber SN. The organization of prefrontal-subthalamic inputs in primates provides an anatomical substrate for both functional specificity and integration: implications for Basal Ganglia models and deep brain stimulation. Journal of Neuroscience. 2013;33:4804–4814. doi: 10.1523/JNEUROSCI.4674-12.2013. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

