Improving the drug-likeness of inspiring natural products - evaluation of the antiparasitic activity against Trypanosoma cruzi through semi-synthetic and simplified analogues of licarin A
- PMID: 32214193
- PMCID: PMC7096397
- DOI: 10.1038/s41598-020-62352-w
Improving the drug-likeness of inspiring natural products - evaluation of the antiparasitic activity against Trypanosoma cruzi through semi-synthetic and simplified analogues of licarin A
Abstract
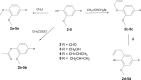
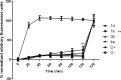
Neolignan licarin A (1) was isolated from leaves of Nectandra oppositifolia (Lauraceae) and displayed activity against trypomastigote forms of the etiologic agent of American trypanosomiasis, Trypanosoma cruzi. Aiming for the establishment of SAR, five different compounds (1a - 1e) were prepared and tested against T. cruzi. The 2-allyl derivative of licarin A (1d) exhibited higher activity against trypomastigotes of T. cruzi (IC50 = 5.0 μM and SI = 9.0), while its heterocyclic derivative 1e displayed IC50 of 10.5 μM and reduced toxicity against NCTC cells (SI > 19.0). However, these compounds presented limited oral bioavailability estimation (<85%, Papp <1.0 × 10-6 cm/s) in parallel artificial membrane permeability assays (PAMPA) due to excessive lipophilicity. Based on these results, different simplified structures of licarin A were designed: vanillin (2), vanillyl alcohol (3), isoeugenol (4), and eugenol (5), as well as its corresponding methyl (a), acetyl (b), O-allyl (c), and C-allyl (d) analogues. Vanillin (2) and its acetyl derivative (2b) displayed expressive activity against intracellular amastigotes of T. cruzi with IC50 values of 5.5 and 5.6 μM, respectively, and reduced toxicity against NCTC cells (CC50 > 200 μM). In addition, these simplified analogues showed a better permeability profile (Papp > 1.0 × 10-6 cm/s) on PAMPA models, resulting in improved drug-likeness. Vanillyl alcohol acetyl derivative (3b) and isoeugenol methyl derivative (4a) displayed activity against the extracellular forms of T. cruzi (trypomastigotes) with IC50 values of 5.1 and 8.8 μM respectively. Based on these results, compounds with higher selectivity index against extracellular forms of the parasite (1d, 1e, 3d, and 4a) were selected for a mechanism of action study. After a short incubation period (1 h) all compounds increased the reactive oxygen species (ROS) levels of trypomastigotes, suggesting cellular oxidative stress. The ATP levels were increased after two hours of incubation, possibly involving a high energy expenditure of the parasite to control the homeostasis. Except for compound 4a, all compounds induced hyperpolarization of mitochondrial membrane potential, demonstrating a mitochondrial imbalance. Considering the unique mitochondria apparatus of T. cruzi and the lethal alterations induced by structurally based on licarin A, these compounds are interesting hits for future drug discovery studies in Chagas disease.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

