The synthetic and therapeutic expedition of isoxazole and its analogs
- PMID: 32214770
- PMCID: PMC7079875
- DOI: 10.1007/s00044-018-2152-6
The synthetic and therapeutic expedition of isoxazole and its analogs
Abstract
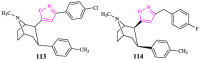
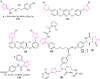
Isoxazole, constituting an important family of five-membered heterocycles with one oxygen atom and one nitrogen atom at adjacent positions is of immense importance because of its wide spectrum of biological activities and therapeutic potential. It is, therefore, of prime importance that the development of new synthetic strategies and designing of new isoxazole derivatives should be based on the most recent knowledge emerging from the latest research. This review is an endeavor to highlight the progress in the chemistry and biological activity of isoxazole derivatives which could provide a low-height flying bird's eye view of isoxazole derivatives to the medicinal chemists for the development of clinically viable drugs using this information.
Keywords: Anti-inflammatory; Anticancer; Antimicrobial; Biological activity; Heterocyclic; Isoxazole.
© Springer Science+Business Media, LLC, part of Springer Nature 2018.
Conflict of interest statement
Conflict of interestThe authors declare that they have no conflict of interest.
Figures



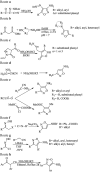

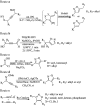

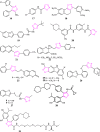





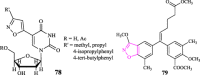









References
-
- Abushanab E, Lee DY, Goodman L. Synthetic studies in the lsoxazolo[4,5- b] pyrazine system. J Heterocycl Chem. 1973;10:181–185. doi: 10.1002/jhet.5570100209. - DOI
-
- Agirbas H, Guner S, Budak F, Keceli S, Kandemirli F, Shvets N, Kovalishyn V, Dimoglo A. Synthesis and structure–antibacterial activity relationship investigation of isomeric 2,3,5-substituted perhydropyrrolo[3,4-d]isoxazole-4,6-diones. Bioorg Med Chem. 2007;15:2322–2333. doi: 10.1016/j.bmc.2007.01.029. - DOI - PubMed
-
- Allegretti PA, Ferreira EM. Platinum-catalyzed cyclizations viacarbene intermediates: syntheses of complementary positional isomers of isoxazoles. Chem Sci. 2013;4:1053–1058. doi: 10.1039/C2SC21671J. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
