Multifunctional Immunoliposomes Combining Catalase and PD-L1 Antibodies Overcome Tumor Hypoxia and Enhance Immunotherapeutic Effects Against Melanoma
- PMID: 32214807
- PMCID: PMC7082626
- DOI: 10.2147/IJN.S225807
Multifunctional Immunoliposomes Combining Catalase and PD-L1 Antibodies Overcome Tumor Hypoxia and Enhance Immunotherapeutic Effects Against Melanoma
Abstract
Background: Immune checkpoint blockades (ICBs) are a promising treatment for cancers such as melanoma by blocking important inhibitory pathways that enable tumor cells to evade immune attack. Programmed death ligand 1 monoclonal antibodies (aPDL1s) can be used as an ICB to significantly enhance the effectiveness of tumor immunotherapy by blocking the PD-1/PD-L1 inhibitory pathway. However, the effectiveness of aPDL1s may be limited by low selectivity in vivo and immunosuppressed tumor microenvironment including hypoxia.
Purpose: To overcome the limitations, we develop a multifunctional immunoliposome, called CAT@aPDL1-SSL, with catalase (CAT) encapsulated inside to overcome tumor hypoxia and aPDL1s modified on the surface to enhance immunotherapeutic effects against melanoma.
Methods: The multifunctional immunoliposomes (CAT@aPDL1-SSLs) are prepared using the film dispersion/post-insertion method. The efficacy of CAT@aPDL1-SSLs is verified by multiple experiments in vivo and in vitro.
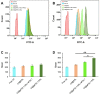
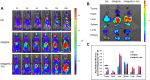
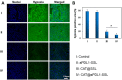
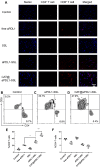
Results: The results of this study suggest that the multifunctional immunoliposomes preserve and protect the enzyme activity of CAT and ameliorate tumor hypoxia. Moreover, the enhanced cellular uptake of CAT@aPDL1-SSLs in vitro and their in vivo biodistribution suggest that CAT@aPDL1-SSLs have great targeting ability,resulting in improved delivery and accumulation of immunoliposomes in tumor tissue.Finally, by activating and increasing the infiltration of CD8+ T cells at the tumor site, CAT@aPDL1-SSLs inhibit the growth of tumor and prolong survival time of mice,with low systemic toxicity.
Conclusion: In conclusion, the multifunctional immunoliposomes developed and proposed in this study are a promising candidate for melanoma immunotherapy, and could potentially be combined with other cancer therapies like radiotherapy and chemotherapy to produce positive outcomes.
Keywords: aPDL1s; immunotherapy; liposomes; melanoma; programmed death ligand 1 monoclonal antibodies; tumor hypoxia.
© 2020 Hei et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

