Functional Comparison between Healthy and Multiple Myeloma Adipose Stromal Cells
- PMID: 32215016
- PMCID: PMC7077052
- DOI: 10.1155/2020/4173578
Functional Comparison between Healthy and Multiple Myeloma Adipose Stromal Cells
Abstract
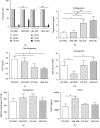
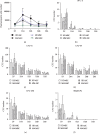
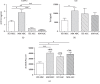
Multiple myeloma (MM) is an incurable B cell neoplasia characterized by the accumulation of tumor plasma cells within the bone marrow (BM). As a consequence, bone osteolytic lesions develop in 80% of patients and remain even after complete disease remission. We and others had demonstrated that BM-derived mesenchymal stromal cells (MSCs) are abnormal in MM and thus cannot be used for autologous treatment to repair bone damage. Adipose stromal cells (ASCs) represent an interesting alternative to MSCs for cellular therapy. Thus, in this study, we wondered whether they could be a good candidate in repairing MM bone lesions. For the first time, we present a transcriptomic, phenotypic, and functional comparison of ASCs from MM patients and healthy donors (HDs) relying on their autologous MSC counterparts. In contrast to MM MSCs, MM ASCs did not exhibit major abnormalities. However, the changes observed in MM ASCs and the supportive property of ASCs on MM cells question their putative and safety uses at an autologous or allogenic level.
Copyright © 2020 Nicolas Espagnolle et al.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this article.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

