Tyrosine kinase inhibitors and immunotherapy combinations in renal cell carcinoma
- PMID: 32215057
- PMCID: PMC7081462
- DOI: 10.1177/1758835920907504
Tyrosine kinase inhibitors and immunotherapy combinations in renal cell carcinoma
Abstract
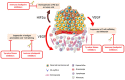
The treatment landscape of metastatic renal cell carcinoma (mRCC) has been transformed with the advent of antiangiogenics, notably tyrosine kinase inhibitors (TKIs) targeting vascular endothelial growth factor receptor (VEGFR), and immune checkpoint inhibitors (ICIs). Both treatment options have improved outcomes of patients and modified the natural history of mRCC. Clinical investigations have focused on evaluating combination regimens containing ICIs and VEGFR-directed TKIs. Namely, the combinations of axitinib plus pembrolizumab (KEYNOTE-426) and axitinib plus avelumab (JAVELIN RENAL 101) have shown improved outcomes compared with sunitinib in treatment-naïve patients with mRCC. In this review, we discuss the clinical data of single-agent TKIs and ICIs in mRCC and the rationale for the combination ICIs and TKIs based on preclinical and clinical evidence. We also explore the current challenges for regimen selection and development of predictive biomarkers.
Keywords: angiogenesis; combination; immune checkpoint inhibitors; immunotherapy; renal cell carcinoma; tyrosine kinase inhibitors.
© The Author(s), 2020.
Conflict of interest statement
Conflict of interest statement: Elie Rassy and Ronan Flippot: none Laurence Albiges: Consulting/advisory role: Novartis (Institution), Amgen (Institution), Bristol-Myers Squibb (Institution), Ipsen (Institution), Roche (Institution), Pfizer (Institution), Astellas Pharma (Institution), Merck (Institution), MSD (Institution), AstraZeneca (Institution), Exelixis (Institution), Corvus Pharmaceuticals (Institution), Peloton therapeutics (Institution). Research Funding: Bristol-Myers Squibb (Institution).
Figures
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. - PubMed
-
- Fisher R, Gore M, Larkin J. Current and future systemic treatments for renal cell carcinoma. Semin Cancer Biol 2013; 23: 38–45. - PubMed
-
- Choueiri TK, Motzer RJ. Systemic therapy for metastatic renal-cell carcinoma. N Engl J Med 2017; 376: 354–366. - PubMed
-
- Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016; 27(Suppl. 5): v58–v68. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources