Dissociation of neonatal and adult mice brain for simultaneous analysis of microglia, astrocytes and infiltrating lymphocytes by flow cytometry
- PMID: 32215337
- PMCID: PMC7090101
- DOI: 10.1016/j.ibror.2019.12.004
Dissociation of neonatal and adult mice brain for simultaneous analysis of microglia, astrocytes and infiltrating lymphocytes by flow cytometry
Abstract
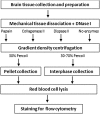
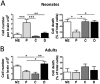
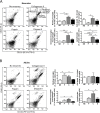
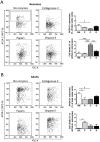
The technical difficulty to isolate microglia, astrocytes and infiltrating immune cells from mouse brain is nowadays a limiting factor in the study of neuroinflammation. Brain isolation requirements are cell-type and animal-age dependent, but current brain dissociation procedures are poorly standardized. This lack of comprehensive studies hampers the selection of optimized methodologies. Thus, we present here a comparative analysis of dissociation methods and Percoll-based separation to identify the most efficient procedure for the combined isolation of healthy microglia, astrocytes and infiltrated leukocytes; distinguishing neonatal and adult mouse brain. Gentle mechanical dissociation and DNase I incubation was supplemented with papain or collagenase II. Dispase II digestion was also used alone or in combination. In addition, cell separation efficiency of 30 % and 30-70 % Percoll gradients was compared. In these experiments, cell yield and integrity of freshly dissociated cells was measured by flow cytometry. We found that papain digestion in combination with dispase II followed by 30 % Percoll separation is the most balanced method to obtain a mixture of microglia, astrocytes and infiltrated immune cells; while addition of dispase II was not an advantage for neonatal brain. These dissociation conditions allowed flow cytometry detection of a slight glial activation triggered by sublethal LPS injection. In conclusion, the enzymes and Percoll density gradients tested here affected differently resting microglia, activated microglia/macrophages, astrocytes and infiltrated lymphocytes. Also, newborn and adult brain showed contrasting reactions to digestion. Our study highlights the strength of flow cytometry for the simultaneous analysis of neuroimmune cell populations once extraction is optimized.
Keywords: ANOVA, one-way analysis of variance; Astrocytes; CNS, Central Nervous System; CaCl2, calcium chloride; EBSS, Earle's Balanced Salt Solution; EDTA, ethylenediaminetetraacetic acid; FACS, Fluorescence-activated cell sorter; FSC, forward-scattered light; Flow cytometry; Glia reactivity; HBSS, Hank's Balanced Salt Solution; LD, lethal dose; LPS, lipopolysaccharide; Lymphocytes; MgCl2, magnesium chloride; MgSO4, magnesium sulfate; Microglia; Neuroimmunity; PBS, phosphate-buffered saline; RT, room temperature; SIP, stock solution of isotonic Percoll; SSC, side-scattered light; i.p, intraperitoneal injection.
© 2020 The Authors.
Figures



References
-
- Bergmann C.C., Altman J.D., Hinton D., Stohlman S.A. Inverted immunodominance and impaired cytolytic function of CD8+ T cells during viral persistence in the central nervous system. J. Immunol. 1999;163:3379–3387. doi: ji_v163n6p3379. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

