Epigenetic Metabolic Reprogramming of Right Ventricular Fibroblasts in Pulmonary Arterial Hypertension: A Pyruvate Dehydrogenase Kinase-Dependent Shift in Mitochondrial Metabolism Promotes Right Ventricular Fibrosis
- PMID: 32216531
- PMCID: PMC7274861
- DOI: 10.1161/CIRCRESAHA.120.316443
Epigenetic Metabolic Reprogramming of Right Ventricular Fibroblasts in Pulmonary Arterial Hypertension: A Pyruvate Dehydrogenase Kinase-Dependent Shift in Mitochondrial Metabolism Promotes Right Ventricular Fibrosis
Abstract
Rationale: Right ventricular (RV) fibrosis in pulmonary arterial hypertension contributes to RV failure. While RV fibrosis reflects changes in the function of resident RV fibroblasts (RVfib), these cells are understudied.
Objective: Examine the role of mitochondrial metabolism of RVfib in RV fibrosis in human and experimental pulmonary arterial hypertension.
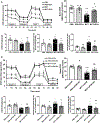
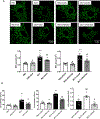
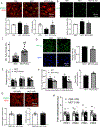
Methods and results: Male Sprague-Dawley rats received monocrotaline (MCT; 60 mg/kg) or saline. Drinking water containing no supplement or the PDK (pyruvate dehydrogenase kinase) inhibitor dichloroacetate was started 7 days post-MCT. At week 4, treadmill testing, echocardiography, and right heart catheterization were performed. The effects of PDK activation on mitochondrial dynamics and metabolism, RVfib proliferation, and collagen production were studied in RVfib in cell culture. Epigenetic mechanisms for persistence of the profibrotic RVfib phenotype in culture were evaluated. PDK expression was also studied in the RVfib of patients with decompensated RV failure (n=11) versus control (n=7). MCT rats developed pulmonary arterial hypertension, RV fibrosis, and RV failure. MCT-RVfib (but not left ventricular fibroblasts) displayed excess mitochondrial fission and had increased expression of PDK isoforms 1 and 3 that persisted for >5 passages in culture. PDK-mediated decreases in pyruvate dehydrogenase activity and oxygen consumption rate were reversed by dichloroacetate (in RVfib and in vivo) or siRNA targeting PDK 1 and 3 (in RVfib). These interventions restored mitochondrial superoxide and hydrogen peroxide production and inactivated HIF (hypoxia-inducible factor)-1α, which was pathologically activated in normoxic MCT-RVfib. Redox-mediated HIF-1α inactivation also decreased the expression of TGF-β1 (transforming growth factor-beta-1) and CTGF (connective tissue growth factor), reduced fibroblast proliferation, and decreased collagen production. HIF-1α activation in MCT-RVfib reflected increased DNMT (DNA methyltransferase) 1 expression, which was associated with a decrease in its regulatory microRNA, miR-148b-3p. In MCT rats, dichloroacetate, at therapeutic levels in the RV, reduced phospho-pyruvate dehydrogenase expression, RV fibrosis, and hypertrophy and improved RV function. In patients with pulmonary arterial hypertension and RV failure, RVfib had increased PDK1 expression.
Conclusions: MCT-RVfib manifest a DNMT1-HIF-1α-PDK-mediated, chamber-specific, metabolic memory that promotes collagen production and RV fibrosis. This epigenetic mitochondrial-metabolic pathway is a potential antifibrotic therapeutic target.
Keywords: DNA methyltransferase 1 (DNMT1); Warburg metabolism; dichloroacetate; hypoxia-inducible factor 1-alpha (HIF-1α); transforming growth factor beta-1 (TGF-β1).
Figures





References
-
- Sandoval J, Bauerle O, Palomar A, Gomez A, Martinez-Guerra ML, Beltran M and Guerrero ML. Survival in primary pulmonary hypertension. Validation of a prognostic equation. Circulation. 1994;89:1733–44. - PubMed
-
- D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT and et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115:343–9. - PubMed
-
- Ghio S, Klersy C, Magrini G, D’Armini AM, Scelsi L, Raineri C, Pasotti M, Serio A, Campana C and Vigano M. Prognostic relevance of the echocardiographic assessment of right ventricular function in patients with idiopathic pulmonary arterial hypertension. International journal of cardiology. 2010;140:272–8. - PubMed
-
- Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation. 2010;122:156–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

