Hemodynamic assessment of diastolic function for experimental models
- PMID: 32216614
- PMCID: PMC7472516
- DOI: 10.1152/ajpheart.00705.2019
Hemodynamic assessment of diastolic function for experimental models
Abstract
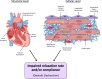
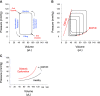
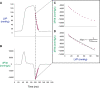
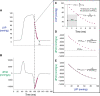
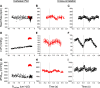
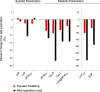
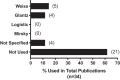
Traditionally, the evaluation of cardiac function has focused on systolic function; however, there is a growing appreciation for the contribution of diastolic function to overall cardiac health. Given the emerging interest in evaluating diastolic function in all models of heart failure, there is a need for sensitivity, accuracy, and precision in the hemodynamic assessment of diastolic function. Hemodynamics measure cardiac pressures in vivo, offering a direct assessment of diastolic function. In this review, we summarize the underlying principles of diastolic function, dividing diastole into two phases: 1) relaxation and 2) filling. We identify parameters used to comprehensively evaluate diastolic function by hemodynamics, clarify how each parameter is obtained, and consider the advantages and limitations associated with each measure. We provide a summary of the sensitivity of each diastolic parameter to loading conditions. Furthermore, we discuss differences that can occur in the accuracy of diastolic and systolic indices when generated by automated software compared with custom software analysis and the magnitude each parameter is influenced during inspiration with healthy breathing and a mild breathing load, commonly expected in heart failure. Finally, we identify key variables to control (e.g., body temperature, anesthetic, sampling rate) when collecting hemodynamic data. This review provides fundamental knowledge for users to succeed in troubleshooting and guidelines for evaluating diastolic function by hemodynamics in experimental models of heart failure.
Keywords: EDP; compliance; guidelines; respiratory; tau.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures









References
-
- Adam RJ, Xia Z, Pravoverov K, Hong J, Case AJ, Schultz HD, Lisco SJ, Zucker IH, Wang HJ. Sympathoexcitation in response to cardiac and pulmonary afferent stimulation of TRPA1 channels is attenuated in rats with chronic heart failure. Am J Physiol Heart Circ Physiol 316: H862–H872, 2019. doi:10.1152/ajpheart.00696.2018. - DOI - PMC - PubMed
-
- Ahmad S, Masjoan Juncos JX, Ahmad A, Zaky A, Wei CC, Bradley WE, Zafar I, Powell P, Mariappan N, Vetal N, Louch WE, Ford DA, Doran SF, Matalon S, Dell’Italia LJ. Bromine inhalation mimics ischemia-reperfusion cardiomyocyte injury and calpain activation in rats. Am J Physiol Heart Circ Physiol 316: H212–H223, 2019. doi:10.1152/ajpheart.00652.2017. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

