A positron emission tomography imaging study to confirm target engagement in the lungs of patients with idiopathic pulmonary fibrosis following a single dose of a novel inhaled αvβ6 integrin inhibitor
- PMID: 32216814
- PMCID: PMC7099768
- DOI: 10.1186/s12931-020-01339-7
A positron emission tomography imaging study to confirm target engagement in the lungs of patients with idiopathic pulmonary fibrosis following a single dose of a novel inhaled αvβ6 integrin inhibitor
Abstract
Background: Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive lung disease with poor prognosis and a significant unmet medical need. This study evaluated the safety, pharmacokinetics (PK) and target engagement in the lungs, of GSK3008348, a novel inhaled alpha-v beta-6 (αvβ6) integrin inhibitor, in participants with IPF.
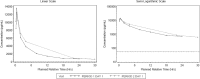
Methods: This was a phase 1b, randomised, double-blind (sponsor unblind) study, conducted in the UK (two clinical sites, one imaging unit) between June 2017 and July 2018 (NCT03069989). Participants with a definite or probable diagnosis of IPF received a single nebulised dose of 1000 mcg GSK3008348 or placebo (ratio 5:2) in two dosing periods. In period 1, safety and PK assessments were performed up to 24 h post-dose; in period 2, after a 7-day to 28-day washout, participants underwent a total of three positron emission tomography (PET) scans: baseline, Day 1 (~ 30 min post-dosing) and Day 2 (~ 24 h post-dosing), using a radiolabelled αvβ6-specific ligand, [18F]FB-A20FMDV2. The primary endpoint was whole lung volume of distribution (VT), not corrected for air volume, at ~ 30 min post-dose compared with pre-dose. The study success criterion, determined using Bayesian analysis, was a posterior probability (true % reduction in VT > 0%) of ≥80%.
Results: Eight participants with IPF were enrolled and seven completed the study. Adjusted posterior median reduction in uncorrected VT at ~ 30 min after GSK3008348 inhalation was 20% (95% CrI: - 9 to 42%). The posterior probability that the true % reduction in VT > 0% was 93%. GSK3008348 was well tolerated with no reports of serious adverse events or clinically significant abnormalities that were attributable to study treatment. PK was successfully characterised showing rapid absorption followed by a multiphasic elimination.
Conclusions: This study demonstrated engagement of the αvβ6 integrin target in the lung following nebulised dosing with GSK3008348 to participants with IPF. To the best of our knowledge this is the first time a target-specific PET radioligand has been used to assess target engagement in the lung, not least for an inhaled drug.
Trial registration: clinicaltrials.gov: NCT03069989; date of registration: 3 March 2017.
Keywords: Alpha-v beta-6; IPF, positron emission tomography; Idiopathic pulmonary fibrosis; Integrin; PET; [18F]FB-A20FMDV2, target engagement; αvβ6.
Conflict of interest statement
TMM has received consultancy fees and, via his institution, has received research funding from GlaxoSmithKline (GSK). JCP received funding from GSK for a clinical fellow but received no personal fees and has no other disclosures. PLM via his institution has received industry-academic funding from AstraZeneca and has received speaker and consultancy fees from Boehringer Ingelheim and Hoffman-La Roche which fall outside the submitted work. GR, GES and AS are employees of Invicro; and GES and AS are former employees of GSK. JKS, FJW, RC, RE, YC, SS, SP, JK, DF, WAH and MVB are employees of and shareholders in GSK. PTL is a former employee of and owns shares in GSK and is now an independent consultant (Target to Treatment Consulting Ltd). RJS, RPM and ARK are former employees of and current shareholders in GSK. JS, LP and PS declare they have no competing interests.
Figures


References
-
- Thomson CC, Duggal A, Bice T, Lederer DJ, Wilson KC, Raghu G. 2018 clinical practice guideline summary for clinicians: diagnosis of idiopathic pulmonary fibrosis. Ann Am Thorac Soc. 2019;16:285–290. - PubMed
-
- Slack RJ, Fisher AJ, Denyer JC, Flint DJ, Pyne S. Quantification of αvβ6 integrin expression in normal and fibrotic human lung tissue. Proc Brit Pharmacol Soc. 2015;13(1):P022.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

