LTBP1 promotes esophageal squamous cell carcinoma progression through epithelial-mesenchymal transition and cancer-associated fibroblasts transformation
- PMID: 32216815
- PMCID: PMC7098101
- DOI: 10.1186/s12967-020-02310-2
LTBP1 promotes esophageal squamous cell carcinoma progression through epithelial-mesenchymal transition and cancer-associated fibroblasts transformation
Abstract
Background: Esophageal squamous cell carcinoma (ESCC) is one of the most prevalent cancers worldwide. Due to its high morbidity and mortality rates, it is urgent to find a molecular target that contributes to esophageal carcinogenesis and progression. In this research, we aimed to investigate the functions of Latent transforming growth factor β binding protein 1(LTBP1) in ESCC progression and elucidate the underlying mechanisms.
Methods: The tandem mass tag-based quantitative proteomic approach was applied to screen the differentially expressed proteins (DEPs) between 3 cases of ESCC tumor samples and paired normal tissues. Then the DEPs were validated in human ESCC tissues using western blot assays and GEPIA database respectively. The expression level of LTBP1 was detected in 152 cases of ESCC tissues and paired normal tissues. Loss-of-function assays were performed to detect the function of LTBP1 in vivo and in vitro. Immunofluorescence and Western blot assays were used to detect the expression of apoptosis, epithelial-mesenchymal transition (EMT) and cancer-associated fibroblasts (CAFs) markers.
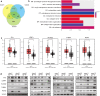
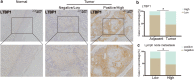
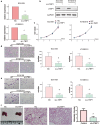
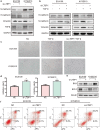
Results: A total of 39 proteins were screened to be up-regulated (ratio > 2.0) in all three ESCC tissues. The results of immunohistochemistry assays indicated that the expression level of LTBP1 was higher in ESCC tissues than that in paired normal tissues (p < 0.001). Overexpression of LTBP1 was positively associated with lymphatic metastasis in ESCC (p = 0.002). Down-regulation of LTBP1 inhibited the invasion and migration as well as metastatic abilities in vitro and in vivo. It was also observed the down-regulation of LTBP1 not only decreased the mesenchymal phenotypes but also inhibited TGFβ-induced EMT in ESCC cells. We further found that down-regulation of LTBP1 enhanced ESCC cells' sensitivity to 5-FU treatment. Inhibition of LTBP1 expression could also attenuate induction of CAFs transformation and restrain fibroblast express fibronectin (FN1) in ESCC cells.
Conclusion: Overexpression of LTBP1 was associated with lymph node metastasis in ESCC. Our results indicated that LTBP1 not only increased the malignant behaviors of ESCC cells but also induced EMT and CAFs transformation. Our studies suggested an oncogenic role of LTBP1 in ESCC progression and it may serve as a potential therapeutic target for ESCC patients.
Keywords: Cancer-associated fibroblasts; Carcinogenesis; Epithelial–mesenchymal transition; Esophageal squamous cell carcinoma; FN1; LTBP1; Metastasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

