Two genomes, one cell: Mitochondrial-nuclear coordination via epigenetic pathways
- PMID: 32217072
- PMCID: PMC7300384
- DOI: 10.1016/j.molmet.2020.01.006
Two genomes, one cell: Mitochondrial-nuclear coordination via epigenetic pathways
Abstract
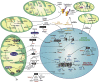
Background: Virtually all eukaryotic cells contain spatially distinct genomes, a single nuclear genome that harbours the vast majority of genes and much smaller genomes found in mitochondria present at thousands of copies per cell. To generate a coordinated gene response to various environmental cues, the genomes must communicate with each another. Much of this bi-directional crosstalk relies on epigenetic processes, including DNA, RNA, and histone modification pathways. Crucially, these pathways, in turn depend on many metabolites generated in specific pools throughout the cell, including the mitochondria. They also involve the transport of metabolites as well as the enzymes that catalyse these modifications between nuclear and mitochondrial genomes.
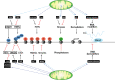
Scope of review: This study examines some of the molecular mechanisms by which metabolites influence the activity of epigenetic enzymes, ultimately affecting gene regulation in response to metabolic cues. We particularly focus on the subcellular localisation of metabolite pools and the crosstalk between mitochondrial and nuclear proteins and RNAs. We consider aspects of mitochondrial-nuclear communication involving histone proteins, and potentially their epigenetic marks, and discuss how nuclear-encoded enzymes regulate mitochondrial function through epitranscriptomic pathways involving various classes of RNA molecules within mitochondria.
Major conclusions: Epigenetic communication between nuclear and mitochondrial genomes occurs at multiple levels, ultimately ensuring a coordinated gene expression response between different genetic environments. Metabolic changes stimulated, for example, by environmental factors, such as diet or physical activity, alter the relative abundances of various metabolites, thereby directly affecting the epigenetic machinery. These pathways, coupled to regulated protein and RNA transport mechanisms, underpin the coordinated gene expression response. Their overall importance to the fitness of a cell is highlighted by the identification of many mutations in the pathways we discuss that have been linked to human disease including cancer.
Keywords: Chromatin; Enzymes; Epigenetics; Histones; Metabolites; Mitochondria; RNA modification.
Copyright © 2020 The Authors. Published by Elsevier GmbH.. All rights reserved.
Figures

References
-
- Xhemalce B., Dawson M.A., Bannister A.J. 2012. Epigenetic regulation and epigenomics in Wiley-Blackwell Advances in molecular biology and medicine; pp. 657–703.
-
- Lyko F. The DNA methyltransferase family: a versatile toolkit for epigenetic regulation. Nature Reviews Genetics. 2017;19:81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

