Rational development of a high-affinity secretin receptor antagonist
- PMID: 32217097
- PMCID: PMC7299832
- DOI: 10.1016/j.bcp.2020.113929
Rational development of a high-affinity secretin receptor antagonist
Abstract
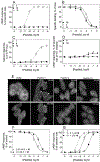
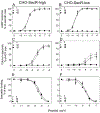
The secretin receptor is a prototypic class B GPCR with substantial and broad pharmacologic importance. The aim of this project was to develop a high affinity selective antagonist as a new and important pharmacologic tool and to aid stabilization of this receptor in an inactive conformation for ultimate structural characterization. Amino-terminal truncation of the natural 27-residue ligand reduced biological activity, but also markedly reduced binding affinity. This was rationally and experimentally overcome with lactam stabilization of helical structure and with replacement of residues with natural and unnatural amino acids. A key new step in this effort was the replacement of peptide residue Leu22 with L-cyclohexylalanine (Cha) to enhance potential hydrophobic interactions with receptor residues Leu31, Val34, and Phe92 that were predicted from molecular modeling. Alanine-replacement mutagenesis of these residues markedly affected ligand binding and biological activity. The optimal antagonist ligand, (Y10,c[E16,K20],I17,Cha22,R25)sec(6-27), exhibited high binding affinity (4 nM), similar to natural secretin, and exhibited no demonstrable biological activity to stimulate cAMP accumulation, intracellular calcium mobilization, or β-arrestin-2 translocation. It acts as an orthosteric competitive antagonist, predicted to bind within the peptide-binding groove in the receptor extracellular domain. The analogous peptide that was one residue longer, retaining Thr5, exhibited partial agonist activity, while further truncation of even a single residue (Phe6) reduced binding affinity. This sec(6-27)-based peptide will be an important new tool for pharmacological and structural studies.
Keywords: Antagonist; G protein-coupled receptor; Secretin; Secretin receptor.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declarations of interest: none.
Figures


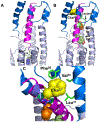


References
-
- Wootten D, Miller LJ, Koole C, Christopoulos A, Sexton PM, Allostery and biased agonism at class B G protein-coupled receptors, Chem. Rev 117 (2017) 111–138. - PubMed
-
- Chey WY, Chang TM, Secretin, 100 years later, J. Gastroenterol 38 (2003) 1025–1035. - PubMed
-
- Dupre J, Rojas L, White JJ, Unger RH, Beck JC, Effects of secretin on insulin and glucagon in portal and peripheral blood in man, Lancet 2 (1966) 26–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

