The inhibitory effect of ECG and EGCG dimeric procyanidins on colorectal cancer cells growth is associated with their actions at lipid rafts and the inhibition of the epidermal growth factor receptor signaling
- PMID: 32217102
- PMCID: PMC7489796
- DOI: 10.1016/j.bcp.2020.113923
The inhibitory effect of ECG and EGCG dimeric procyanidins on colorectal cancer cells growth is associated with their actions at lipid rafts and the inhibition of the epidermal growth factor receptor signaling
Abstract
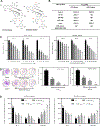
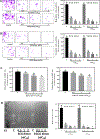
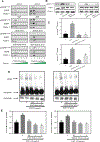
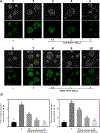
Colorectal cancer (CRC) is one of the most common cancers worldwide. Epidemiological studies indicate that consumption of fruits and vegetables containing procyanidins is associated with lower CRC risk. This study investigated the capacity of two dimeric procyanidins composed of epicatechin gallate (ECG) or epigallocatechin gallate (EGCG) isolated from persimmons, to inhibit CRC cell growth and promote apoptosis, characterizing the underlying mechanisms. ECG and EGCG dimers reduced the growth of five human CRC cell lines in a concentration (10-60 μM)- and time (24-72 h)-dependent manner, with a 72 h-IC50 value in Caco-2 cells of 10 and 30 μM, respectively. ECG and EGCG dimers inhibited Caco-2 cell proliferation by arresting the cell cycle in G2/M phase and by inducing apoptosis via the mitochondrial pathway. In addition, ECG and EGCG dimers inhibited cell migration, invasion, and adhesion, decreasing the activity of matrix metalloproteinases (MMP-2/9). Mechanistically, ECG and EGCG dimers inhibited the activation of lipid raft-associated epidermal growth factor (EGF) receptor (EGFR), without affecting its localization at lipid rafts. In particular, ECG and EGCG dimers reduced EGFR phosphorylation at Tyr1068 residue, prevented EGFR dimerization and activation upon stimulation, and induced EGFR internalization both in the absence and presence of EGF. Furthermore, ECG and EGCG dimers increased EGFR phosphorylation at Tyr1045 residue, providing a docking site for ubiquitin ligase c-Cbl and induced EGFR degradation by the proteasome. Downstream of EGFR, ECG and EGCG dimers inhibited the activation of the MEK/ERK1/2 and PI3K/AKT signaling pathways, downregulating proteins involved in the modulation of cell survival. In conclusion, ECG and EGCG dimers reduced CRC cell growth by inhibiting EGFR activation at multiple steps, including the disruption of lipid rafts integrity and promoting EGFR degradation. These results shed light on a potential molecular mechanism on how procyanidins-rich diets may lower CRC risk.
Keywords: Apoptosis; Colorectal cancer; ECG and EGCG dimers; EGFR signaling; Lipid rafts.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Adachi S, et al. (2007). “The inhibitory effect of (−)-epigallocatechin gallate on activation of the epidermal growth factor receptor is associated with altered lipid order in HT29 colon cancer cells.” Cancer Research 67(13): 6493–6501. - PubMed
-
- Arnold M, et al. (2017). “Global patterns and trends in colorectal cancer incidence and mortality.” Gut 66(4): 683–691. - PubMed
-
- Aune D, et al. (2011). “Nonlinear Reduction in Risk for Colorectal Cancer by Fruit and Vegetable Intake Based on Meta-analysis of Prospective Studies.” Gastroenterology 141(1): 106–118. - PubMed
-
- Baena R and Salinas P (2015). “Diet and colorectal cancer.” Maturitas 80(3): 258–264. - PubMed
-
- Ben QW, et al. (2014). “Dietary Fiber Intake Reduces Risk for Colorectal Adenoma: A Meta-analysis.” Gastroenterology 146(3): 689–+. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

