The natural history of progressive fibrosing interstitial lung diseases
- PMID: 32217654
- PMCID: PMC7315005
- DOI: 10.1183/13993003.00085-2020
The natural history of progressive fibrosing interstitial lung diseases
Abstract
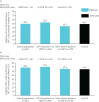
We used data from the INBUILD and INPULSIS trials to investigate the natural history of progressive fibrosing interstitial lung diseases (ILDs).Subjects in the two INPULSIS trials had a clinical diagnosis of idiopathic pulmonary fibrosis (IPF) while subjects in the INBUILD trial had a progressive fibrosing ILD other than IPF and met protocol-defined criteria for ILD progression despite management. Using data from the placebo groups, we compared the rate of decline in forced vital capacity (FVC) (mL·year-1) and mortality over 52 weeks in the INBUILD trial with pooled data from the INPULSIS trials.The adjusted mean annual rate of decline in FVC in the INBUILD trial (n=331) was similar to that observed in the INPULSIS trials (n=423) (-192.9 mL·year-1 and -221.0 mL·year-1, respectively; nominal p-value=0.19). The proportion of subjects who had a relative decline in FVC >10% predicted at Week 52 was 48.9% in the INBUILD trial and 48.7% in the INPULSIS trials, and the proportion who died over 52 weeks was 5.1% in the INBUILD trial and 7.8% in the INPULSIS trials. A relative decline in FVC >10% predicted was associated with an increased risk of death in the INBUILD trial (hazard ratio 3.64) and the INPULSIS trials (hazard ratio 3.95).These findings indicate that patients with fibrosing ILDs other than IPF, who are progressing despite management, have a subsequent clinical course similar to patients with untreated IPF, with a high risk of further ILD progression and early mortality.
Trial registration: ClinicalTrials.gov NCT02999178 NCT01335464 NCT01335477.
Copyright ©ERS 2020.
Conflict of interest statement
Conflict of interest: K.K. Brown reports grants from NHLBI; personal fees from Biogen, Blade Therapeutics, Galapagos, Galecto Biotech, Huitai Biomedicine, Lifemax, Lilly, MedImmune, monARC Bionetworks, Pliant Therapeutics, ProMetic, Third Pole Therapeutics, Theravance, Three Lakes Partners, and Veracyte; personal fees and non-financial support from Boehringer Ingelheim; and other support from Genoa and the Open Source Imaging Consortium (OSIC). Conflict of interest: F.J. Martinez reports grants, personal fees, non-financial support and other support from Boehringer Ingelheim; personal fees, nonfinancial support and other support from AstraZeneca; non-financial support and other support from ProterixBio; personal fees and non-financial support from the Canadian Respiratory Network, Chiesi, CME Outfitters, Dartmouth, Genentech, GlaxoSmithKline, Inova Fairfax Health System, Miller Communications, the National Association for Continuing Education, Novartis, Pearl Pharmaceuticals, PeerView Communications, Physicians Education Resource, Potomac, Prime Communications, the Puerto Rican Respiratory Society, Sunovion, Teva, Theravance, the University of Alabama Birmingham, and Vindico; personal fees and other support from Patara/Respivant; grants from NIH; personal fees from the American Thoracic Society, Columbia University, France Foundation, MD Magazine, Methodist Hospital Brooklyn, New York University, Physicians Education Resource, Rare Disease Healthcare Communications, Rockpointe, UpToDate, and WebMD/Medscape; other support from Afferent/Merck, Bayer, Biogen, Bridge Biotherapeutics, Gala Pharmaceutical, Promedior, Wolters Kluwer, and Veracyte; and non-financial support from Gilead, Nitto, Prometic, and Zambon. Conflict of interest: S.L.F. Walsh reports personal fees for consultancy from Sanofi-Aventis, Galapagos and OSIC, personal fees for advisory board work from Roche, grants and personal fees for steering committee work from Boehringer Ingelheim, personal fees for lectures from Bracco, outside the submitted work. Conflict of interest: V.J. Thannickal reports personal fees for consultancy from Boehringer Ingelheim Pharmaceuticals, Inc., Kadmon Corporation, Pliant, Glenmark, Covance, Blade, Versant Venture, Mistral and Translate Bio, grants from Genkyotex, outside the submitted work. Conflict of interest: A. Prasse reports that Hannover Medical School received a fee for patient randomisation into the INBUILD study from Boehringer Ingelheim; personal fees for consultancy and lectures and non-financial support (travel expenses) from Boehringer Ingelheim and Roche, personal fees for lectures and non-financial support (travel expenses) from Novartis, AstraZeneca and Chiesi, personal fees for consultancy and non-financial support (travel expenses) from Nitto Denko and Pliant, outside the submitted work. Conflict of interest: R. Schlenker-Herceg is an employee of Boehringer Ingelheim Pharmaceuticals, Inc. Conflict of interest: R-G. Goeldner is an employee of Boehringer Ingelheim Pharma GmbH & Co., KG. Conflict of interest: E. Clerisme-Beaty is an employee of Boehringer Ingelheim International GmbH. Conflict of interest: K. Tetzlaff is an employee of Boehringer Ingelheim International GmbH. Conflict of interest: V. Cottin reports personal fees for advisory board work and lectures, and non-financial support for meeting attendance from Actelion, grants, personal fees for advisory board work and lectures, and non-financial support for meeting attendance from Boehringer Ingelheim and Roche, personal fees for advisory board work and data monitoring committee work from Bayer/MSD, Promedior and Galapagos, personal fees for adjudication committee work from Gilead, personal fees for advisory board work and lectures from Novartis, personal fees for lectures from Sanofi, personal fees for data monitoring committee work from Celgene and Galecto, outside the submitted work. Conflict of interest: A.U. Wells reports personal fees for consultancy and lectures from Boehringer Ingelheim and Roche, personal fees for consultancy from Blade, outside the submitted work.
Figures



Comment in
-
Progressive fibrosing interstitial lung disease: we know it behaves badly, but what does that mean?Eur Respir J. 2020 Jun 25;55(6):2000894. doi: 10.1183/13993003.00894-2020. Print 2020 Jun. Eur Respir J. 2020. PMID: 32586840 No abstract available.
-
June Podcast: The natural history of progressive fibrosing ILD.Eur Respir J. 2020 Jul 7;55(6):20E5506. doi: 10.1183/13993003.E5506-2020. Print 2020 Jun. Eur Respir J. 2020. PMID: 32636184 No abstract available.
-
Comment on "The natural history of progressive fibrosing interstitial lung diseases".Eur Respir J. 2020 Dec 17;56(6):2003508. doi: 10.1183/13993003.03508-2020. Print 2020 Dec. Eur Respir J. 2020. PMID: 33334778 No abstract available.
-
Reply to comment on "The natural history of progressive fibrosing interstitial lung diseases".Eur Respir J. 2020 Dec 17;56(6):2003967. doi: 10.1183/13993003.03967-2020. Print 2020 Dec. Eur Respir J. 2020. PMID: 33334779 Free PMC article.
References
-
- Raghu G, Remy-Jardin M, Myers JL, et al. . Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2018; 198: e44–e68. - PubMed
-
- Wells AU, Brown KK, Flaherty KR, et al. . What's in a name? That which we call IPF, by any other name would act the same. Eur Respir J 2018; 51: 1800692. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
