Evaluating the potential of hyperpolarised [1-13C] L-lactate as a neuroprotectant metabolic biosensor for stroke
- PMID: 32218474
- PMCID: PMC7099080
- DOI: 10.1038/s41598-020-62319-x
Evaluating the potential of hyperpolarised [1-13C] L-lactate as a neuroprotectant metabolic biosensor for stroke
Abstract
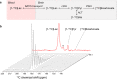
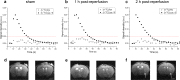
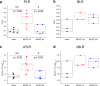
Cerebral metabolism, which can be monitored by magnetic resonance spectroscopy (MRS), changes rapidly after brain ischaemic injury. Hyperpolarisation techniques boost 13C MRS sensitivity by several orders of magnitude, thereby enabling in vivo monitoring of biochemical transformations of hyperpolarised (HP) 13C-labelled precursors with a time resolution of seconds. The exogenous administration of the metabolite L-lactate was shown to decrease lesion size and ameliorate neurological outcome in preclinical studies in rodent stroke models, as well as influencing brain metabolism in clinical pilot studies of acute brain injury patients. The aim of this study was to demonstrate the feasibility of measuring HP [1-13C] L-lactate metabolism in real-time in the mouse brain after ischaemic stroke when administered after reperfusion at a therapeutic dose. We showed a rapid, time-after-reperfusion-dependent conversion of [1-13C] L-lactate to [1-13C] pyruvate and [13C] bicarbonate that brings new insights into the neuroprotection mechanism of L-lactate. Moreover, this study paves the way for the use of HP [1-13C] L-lactate as a sensitive molecular-imaging biosensor in ischaemic stroke patients after endovascular clot removal.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Sandercock P, et al. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): a randomised controlled trial. Lancet. 2012;379:2352–2363. doi: 10.1016/S0140-6736(12)60768-5. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

