State-of-the-art neonatal cerebral ultrasound: technique and reporting
- PMID: 32218539
- PMCID: PMC7098885
- DOI: 10.1038/s41390-020-0776-y
State-of-the-art neonatal cerebral ultrasound: technique and reporting
Abstract
In the past three decades, cerebral ultrasound (CUS) has become a trusted technique to study the neonatal brain. It is a relatively cheap, non-invasive, bedside neuroimaging method available in nearly every hospital. Traditionally, CUS was used to detect major abnormalities, such as intraventricular hemorrhage (IVH), periventricular hemorrhagic infarction, post-hemorrhagic ventricular dilatation, and (cystic) periventricular leukomalacia (cPVL). The use of different acoustic windows, such as the mastoid and posterior fontanel, and ongoing technological developments, allows for recognizing other lesion patterns (e.g., cerebellar hemorrhage, perforator stroke, developmental venous anomaly). The CUS technique is still being improved with the use of higher transducer frequencies (7.5-18 MHz), 3D applications, advances in vascular imaging (e.g. ultrafast plane wave imaging), and improved B-mode image processing. Nevertheless, the helpfulness of CUS still highly depends on observer skills, knowledge, and experience. In this special article, we discuss how to perform a dedicated state-of-the-art neonatal CUS, and we provide suggestions for structured reporting and quality assessment.
Conflict of interest statement
The authors declare no competing interests.
Figures
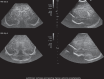
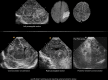


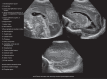


References
-
- Meijler, G. Neonatal Cranial Ultrasonography 2nd edn (Springer, Berlin, Heidelberg, 2012).
-
- Plaisier, A. et al. Serial cranial ultrasonography or early MRI for detecting preterm brain injury? Arch. Dis. Child. Fetal Neonatal Ed. 100, F293–F300 (2015). - PubMed

